COVID-19 のパンデミックをきっかけに、米国、欧州をはじめとした世界中で行われた都市封鎖対策に対応すべく、施設は迅速に業務を調整しなければなりませんでした。メディデータは、この新たな現実におけるパンデミックの影響と治験責任医師のニーズをより深く理解するために、世界の治験実施施設を対象とした調査を実施しました。施設の関係者はメディデータのプラットフォームにおける最大のユーザーグループであり、パンデミックが臨床試験に与える影響について現場の貴重な意見やインサイトを提供してくれました。(本調査にご協力をいただきました関係者の皆様に深く感謝申し上げます。)
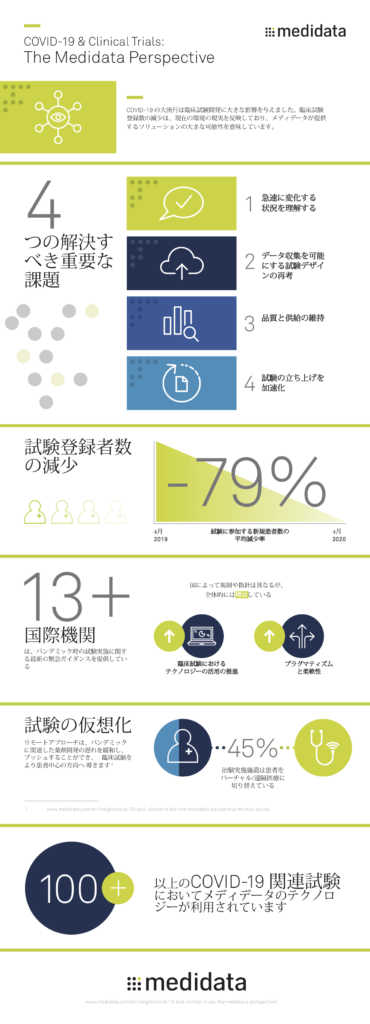
当然のことながら、回答者は圧倒的にパンデミックを試験実施上の重要な要因として認識しています。回答者の69%がCOVID-19が進行中の試験の継続能力に影響を与えており、78%の回答者がCOVID-19が新しい試験の開始に影響を与えていると報告しています。これらの回答は、パンデミックの影響のペースと大きさに合わせて、適応可能な運営モデルとソリューションを必要としていることを示しています。多くの施設では、スポンサーやCROに対して、従来のビジネスルールでは業務を遂行することができないという事実を理解してもらう必要があると考えています。
最大の懸念事項:患者の募集と登録
COVID-19の試験関連活動への影響を見てみると、回答者は患者の募集と登録に関する懸念を上位2つの課題として挙げています。多くの施設では、都市封鎖、移動制限、健康上の懸念により、患者や施設スタッフを招集することが困難または不可能となっています。すでに、調査回答者の3分の2以上が進行中の試験での患者募集を中止しているか、または間もなく中止する予定であり、3分の1が無作為化を中止しており、約半数が試験を延期しているか、または延期する予定であると回答しています。
治験実施施設の回復力と適応力
COVID-19が提示した課題にもかかわらず、施設は患者の安全性を維持し、パンデミックの試験実施への影響を軽減するために新しいアプローチを採用することで、回復力と創意工夫を示しています。特筆すべきは、COVID-19に対応するために実施している措置を施設に尋ねたところ、半数以上の施設が施設内での患者訪問をバーチャルな形式に変更したり、患者との対話のために遠隔医療を利用し始めたりしています。さらに、40%以上の施設が、治験薬を直接患者に発送しています。この危機を管理するために施設がリアルタイムで変更を実施する際には、スポンサーやCROからのポリシーとサポートの両方が必要と考えています。
最前線からの提言
スポンサーや医薬品開発業務受託機関(CRO)への提言を求められた際、各施設からは、「ワンサイズ・フィット・オール」のような万人に適用する従来のやり方を検証し、プロトコルの逸脱に対する柔軟性と理解を高め、施設の対応能力を高めること、施設を訪れることができない患者に対応するための遠隔医療の採用、追加の財政支援、将来のアウトブレイクに備えた試験固有の緊急時対応計画の策定などが提案されました。
これらの提案は、COVID-19の時代に試験を成功させるために必要な基本的な運営上の変更を反映している。パンデミックによって物理的な施設と従来の臨床運営に生じた制限は、バーチャル試験と分散型の患者参加をサポートする技術の必要性を大きく加速させています。
本記事は2020年5月20日に投稿された英文ブログ記事の抄訳となります。原文はこちらからご覧ください。
