eCOA
Faster study start-up, while building rich patient experiences
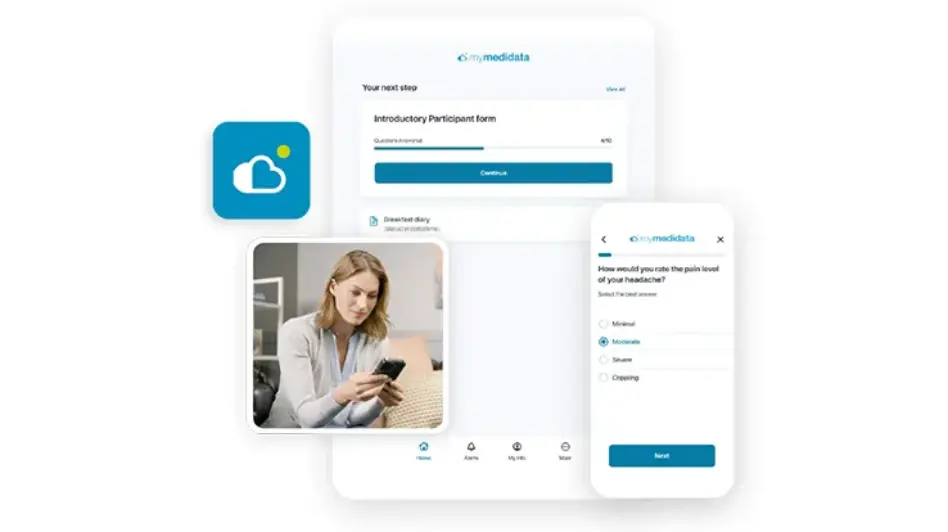
eCOA (Clinical Outcome Assessment) is revolutionizing the way sponsors, CROs, and sites collect electronic data from patients, physicians, and caregivers. Medidata’s eCOA capability is built using Designer, enabling Sponsors and CRO partners to build rich patient experiences via intuitive drag and drop screen templates and visual workflow tools. Available through myMedidata, patients can easily access their study tasks through the web or an app on any mobile device.
Built as part of the unified Medidata Platform, Medidata’s eCOA improves your study experience with flexible deployment options, a groundbreaking global instrument library, and dedicated services and support.
Image Capture Directly Into eCOA Forms
Combining the power of eCOA with Rave Imaging, eCOA Image Capture enables patients and caregivers to take and share pictures from their mobile devices and upload them directly into the eCOA, eliminating the need for extra site visits or multiple technology solutions. Images become available for immediate review, delivering greater insights for both sites and sponsors, while enhancing the patient experience. This patient-centric feature also enhances the use of eCOA in hybrid and decentralized clinical trials.
The Medidata eCOA Difference
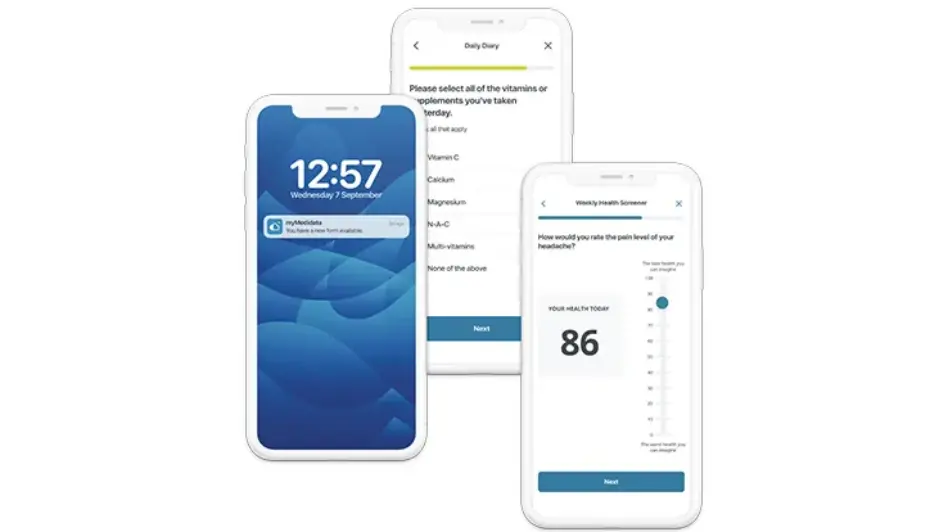
Significantly Cut Study Build Times
Powered by the new Designer tool, gain flexibility in customizing all aspects of eCOA using a library of approved and reusable forms leading to time savings of up to 50% on study builds. As part of a unified platform with a single point of data entry, there are fewer queries and cleaner data with no end-of-study mapping or integration required.

Unified Platform Approach
The Medidata Platform provides a clear view of all your cross-application data in one place. Integrate with your existing data systems, eliminating manual data entry and reconciliation, while maintaining full control and oversight.
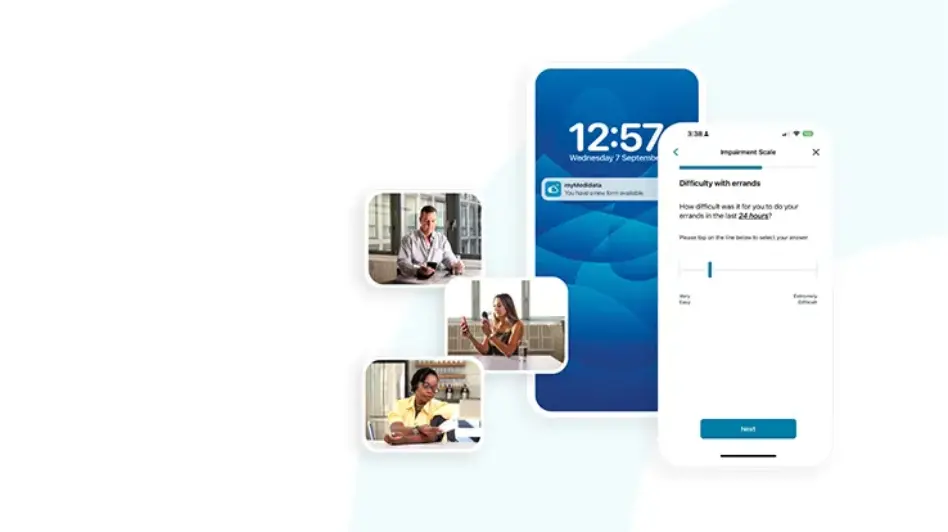
Patient-Centric and Easy to Use
Offer patients the flexibility of choice in how they participate with the ability to engage in trial activities while at home and/or on the go. Information syncs immediately into the clinical dataset, allowing real time visibility. Enhancing the patient journey is a dedicated Patient Cloud Helpdesk, easing patient burden and making decentralized clinical trials a reality.
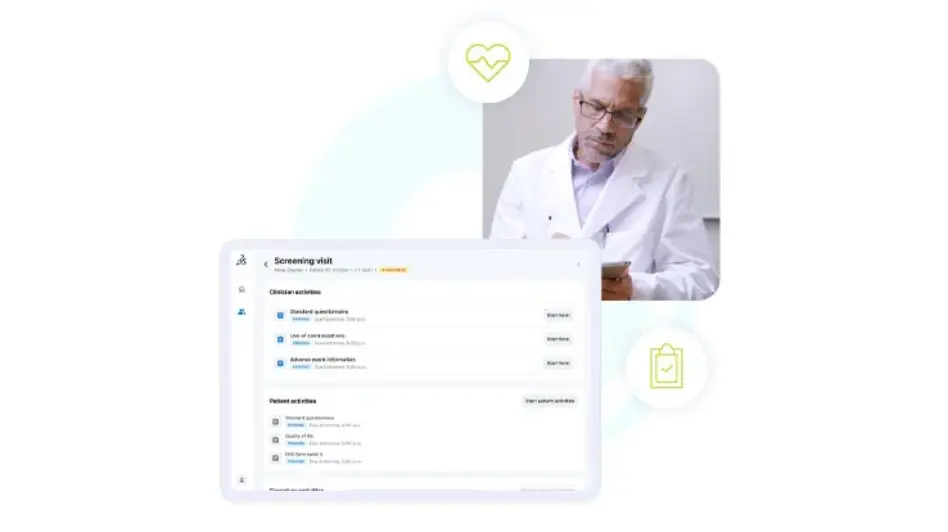
Integrated Site Option
Designed to harness the power of Medidata Designer and work seamlessly with the myMedidata App for a complete trial experience, Medidata App offers a user-friendly platform for efficient eCOA data collection at sites, as well as patient management and eSource capabilities.
Key Features of Medidata eCOA
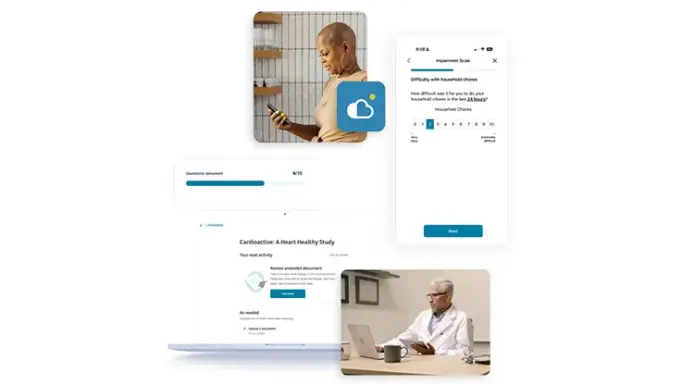
Multiple Ways to Engage
Simplify clinical trial activities by giving patients one login to access all of their trial tasks through their myMedidata account from a native app or any web-enabled device in any location.
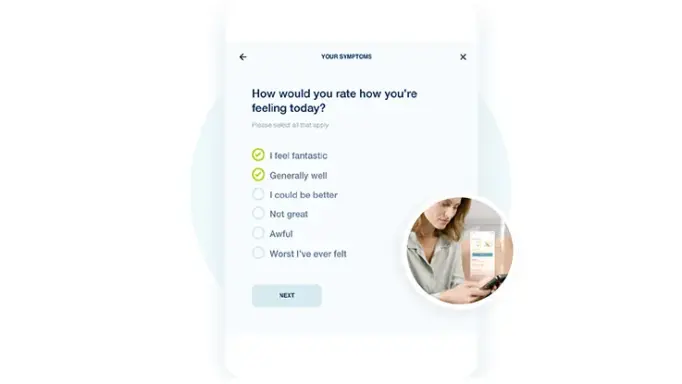
Upgrade your Patients’ Experiences
Build more visual experiences and provide better context for patients with custom image scales, dosing instructions, and reference images included directly in forms.
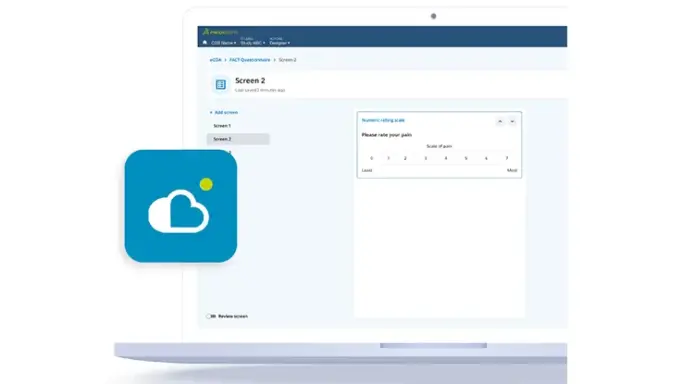
Industry Transforming eCOA Global Library
Create richer and more capable eCOA forms with access to a global library of reusable and validated instruments, including translations, author agreements, and multiple configurable options.
Related Solutions
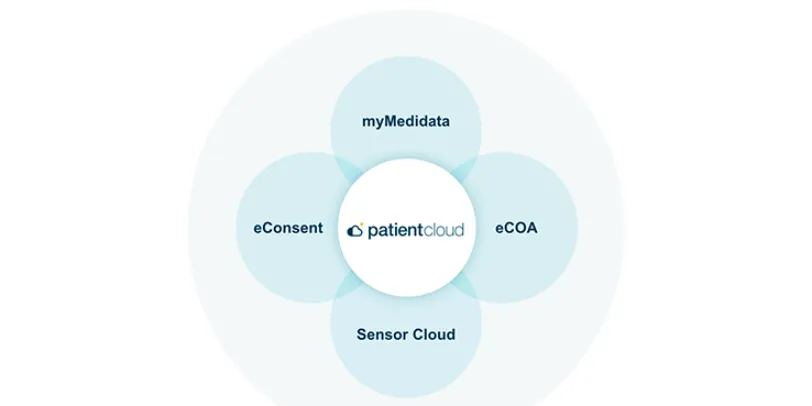
Patient Cloud
Patient Cloud is a suite of powerful solutions that makes it simple and engaging for patients to participate in any clinical trial – so your trials are easier, faster, and produce better results.
Sensor Cloud
Medidata Sensor Cloud takes a unique approach to managing a broad range of wearable sensor and digital health technology data for clinical trials. Our common data model and proprietary algorithms enable rapid ingestion and analysis of patient data resulting in faster, better decisions for a more flexible patient experience.
myMedidata
myMedidata is a clinical research portal-for-life. Accessed using any web-enabled device, patients can use myMedidata to find, learn about, enroll and participate in all clinical trial activities from a single user interface.
Learn More

Advancing Decentralized Clinical Trials Through a Unified Approach to eCOA and Digital Health Technologies
Drug developers are increasingly harnessing the combined power of objective data measured using digital health technologies (DHTs) and subjective clinical outcome assessments that include patient-reported outcomes (PROs).
Uniting these two complementary mechanisms is enabling the development of comprehensive patient profiles that better inform how patients experience their disease and respond to treatment.

Using eCOA in Oncology Clinical Trials
Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment” by lead study author Ethan M. Basch, MD, MSc, FASCO, and his clinical data management team.

Biotech Delivers a Superior Patient and Site Experience with the Medidata Platform
Learn how a small biotech company turned to Medidata to make the best use of limited funds and time. Implementing Medidata Platform reduced study startup time, improved study compliance, and delivered a superior patient and site experience while streamlining communication between all trial stakeholders.

Leading Pharma Company Takes A Unified Approach to Streamline Processes, Speed Study Start Up, and Improve Data Quality
Discover how Medidata eCOA helped to motivate patients and increased engagement around experiences outside of the clinical setting, which otherwise may have gone unmentioned.

