myMedidata
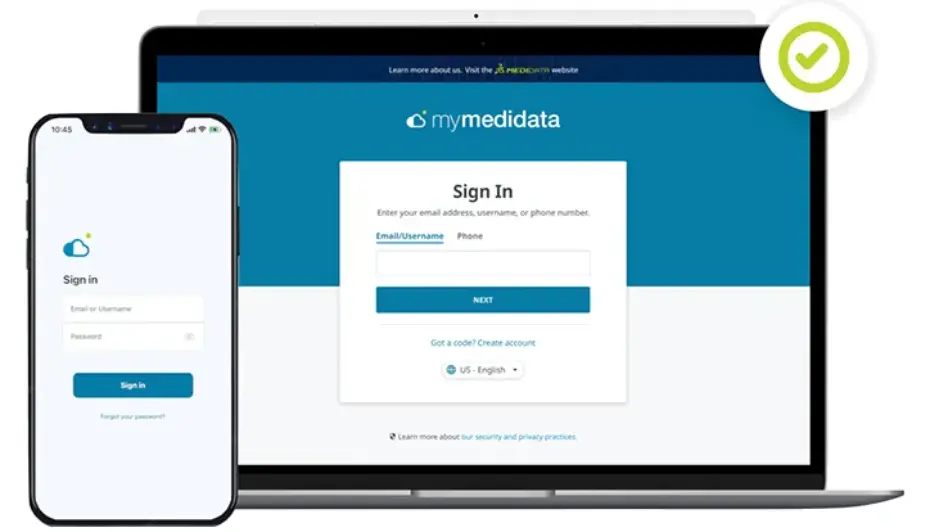
Built for patients, by patients, myMedidata is a single destination patient portal, allowing patients to use any online device to virtually learn, enroll and engage in clinical trials.
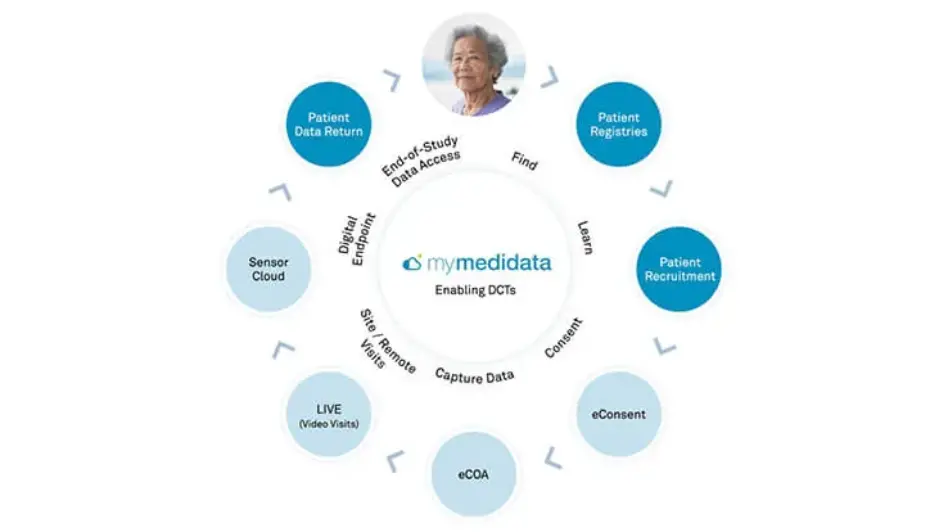
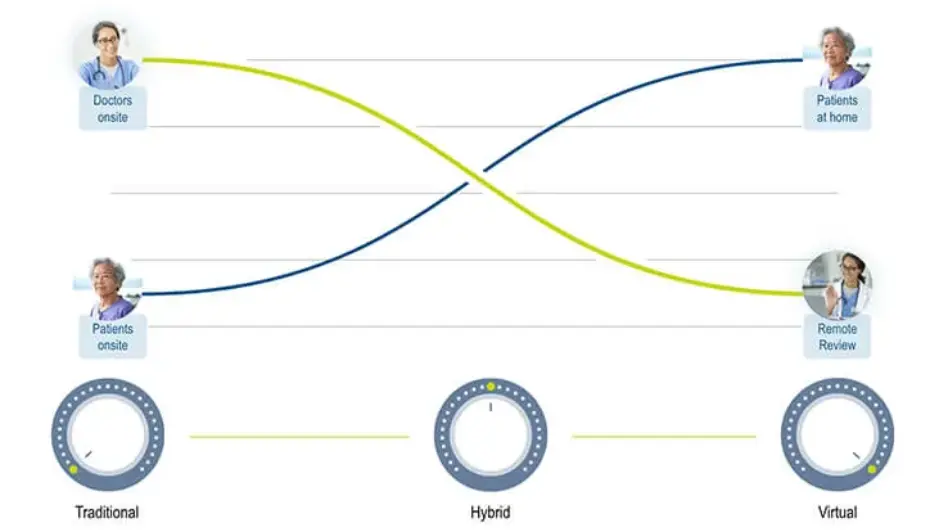
Only myMedidata enables the full range of tools to build scalable, flexible solutions at every level of decentralized and hybrid clinical trials and our expert teams help you tackle problems creatively to find the most effective level of decentralization.
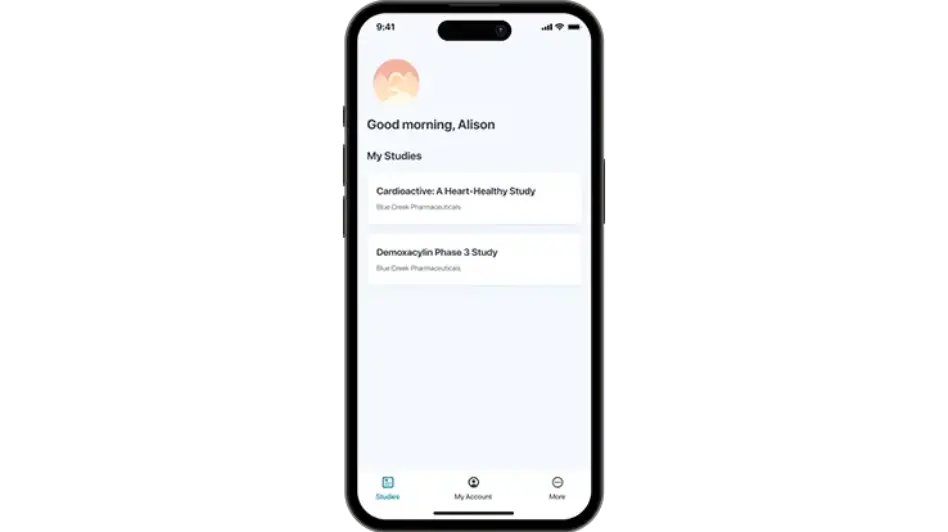
myMedidata App
Expedite study start up and improve patient experiences. With this powerful native app, patients now have an additional option to easily access their myMedidata patient portal through any mobile device – integrating clinical trial activities seamlessly into daily life.
The myMedidata App is built using Medidata Designer, our new platform configuration tool that builds rich patient experiences through easy to use screen templates, drastically reducing study start-up time.
myMedidata app is available on iOS and Android, and can be used both on the patients’ own devices (BYOD) or via a provisioned device.
Put Patients at the Forefront of Your Studies
Single Clinical Trial Dashboard for Life
Eliminate the need for extra apps, logins, and unnecessary provisioned devices by giving your patients one location for all of their clinical trial activities. myMedidata makes it simple and engaging for patients to participate in any clinical trial so your trials are easier, faster, and produce better results.

Enables Continuous Clinical Data Capture
Empower patients with choice in how they participate in research, so you can efficiently recruit the widest, most inclusive pool of participants, keep them engaged throughout your trial, and produce better study results. Only Medidata allows clinical trials to be more efficient with sites and patients on the same data platform, eliminating integrations and reducing burdens for patients and site staff.
Standardize on Technology
myMedidata comes unified with the Medidata Platform, the platform used by the majority of clinical trials in the world- eliminating the need for workflows, expediting timelines, mitigating risk, and reducing burdens. Only Medidata offers a scalable, end-to-end platform with easy configuration and multiple delivery models based on needs.
Key Features of myMedidata

Clearer Consenting Process
To virtually enroll in a new study, patients access their myMedidata account, then are guided through an electronic consenting process, known as eConsent. Through myMedidata eConsent, patients watch the study’s eConsent video and review all relevant consent documents. Upon confirming full understanding of the consent, the patient virtually signs their web-based eConsent. myMedidata eConsent can be paired with myMedidata LIVE Video Visits to allow for additional communications and touchpoints between study site staff and participants.
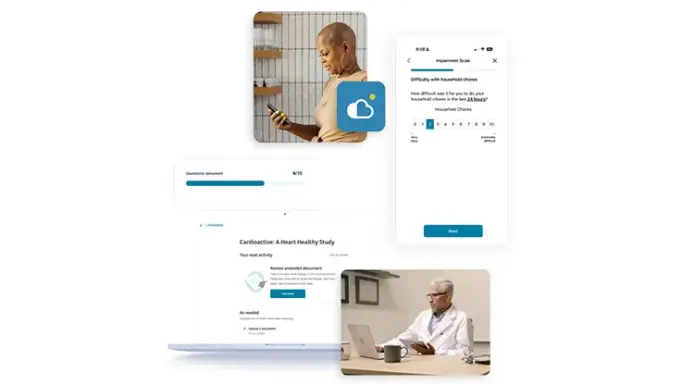
Upgraded Patient Experience
eCOA is built using Designer, enabling Sponsors and CRO partners to build rich patient experiences via intuitive visual workflow tools. Available through the myMedidata native mobile app or any web-enabled device, patients can conveniently access their study tasks where and when it’s best for them. A better overall study experience makes it easier for patients to stay enrolled in trials while expediting timelines and reducing overall costs for sites and sponsors.
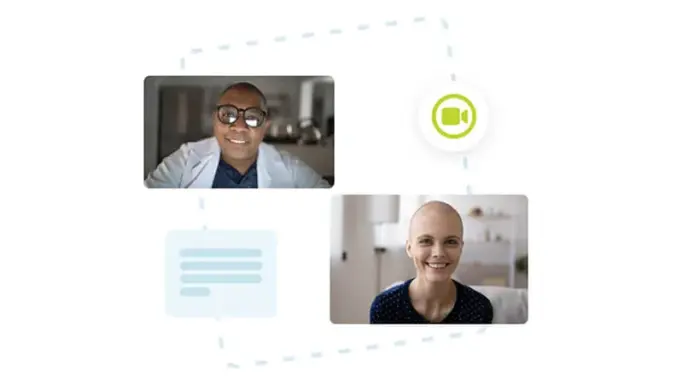
Video Visits / Telehealth
myMedidata LIVE is a web-based, live video conferencing capability, virtually connecting patients with their clinical trial study staff. A myMedidata LIVE video visit between patients and sites can replace a scheduled site-based appointment and allow the study teams to complete their data entry in Rave while the patient remains engaged offsite through myMedidata. When used in combination with myMedidata Registries and eConsent, site staff and patients can remain connected without the need for additional travel burdens.
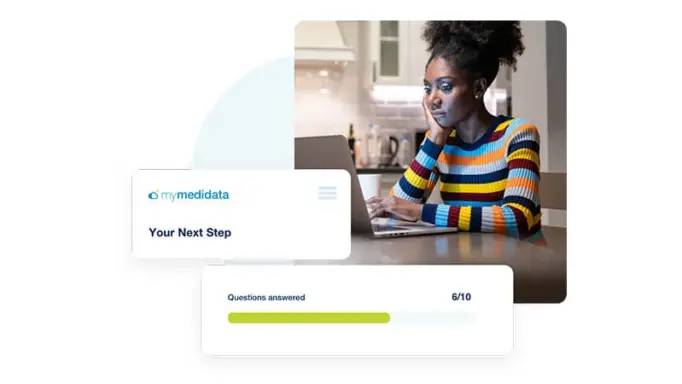
Increased Trial Engagement
myMedidata Registries expands patient participation from a single trial transaction to pre-and post-trial engagement and patient data return, resulting in a community of educated, empowered, and engaged patients prepared to participate.

Built for Patients by Patients
Medidata’s Patient Insights program infuses the patient perspective into the software development life cycle to create technical solutions that improve the overall patient experience in clinical research operations. The most sophisticated innovations in clinical trial technology are meaningless if patients won’t use them. To improve patient engagement, Medidata has now expanded access to our Patient Insights Board (PIB) and award-winning Patient Centricity by Design methodology to allow sponsors and CROs to optimize trial design and patient participation.
Related Solutions
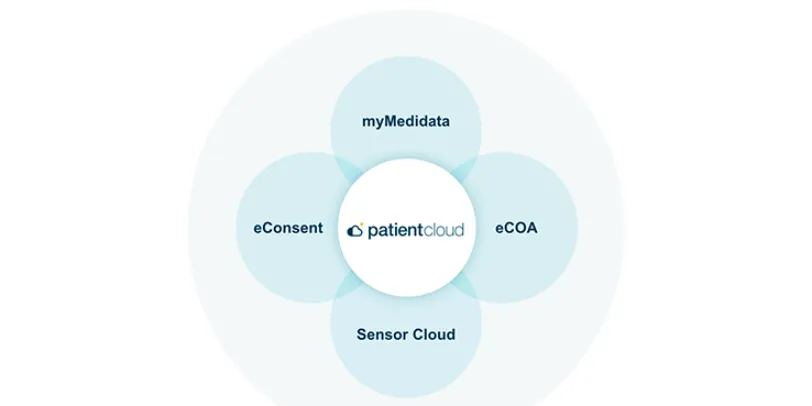
Medidata Patient Cloud
Patient Cloud is a suite of powerful solutions that makes it simple and engaging for patients to participate in any clinical trial – so your trials are easier, faster, and produce better results.
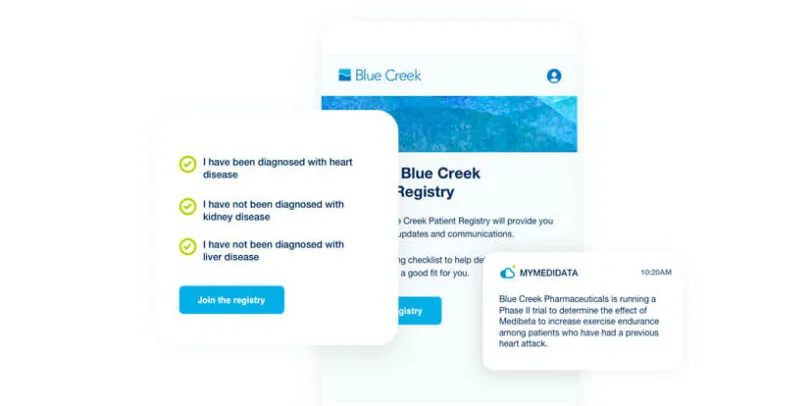
myMedidata Registries
myMedidata Registries helps patients learn more about clinical trial opportunities by expanding patient participation from a single trial transaction to pre-and post-trial engagement, resulting in a community of educated, empowered, and engaged patients.

Decentralized Clinical Trials
With our expanded Decentralized Clinical Trials Program, Medidata is delivering the industry’s only scalable, end-to-end offering for trial decentralization that connects trial experiences for patients, sites, and sponsors.

Patient Insights
Our Patient Centricity by Design initiative is built around three core principles of patient centricity: design, engagement, and activation.
Each Patient Cloud product is designed using Patient Insights generated from our Patient Design Studios – allowing patients to become active participants in the development of solutions for clinical trials.
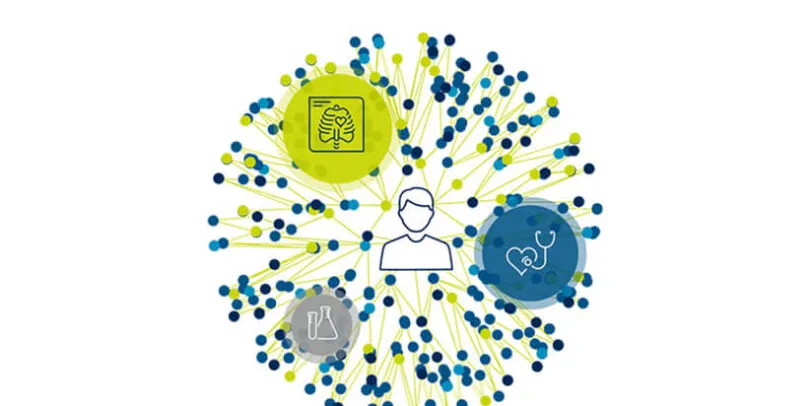
Medidata Link
Medidata Link accelerates insights within & beyond the clinical trial by allowing Sponsors or CROs to connect patient-level clinical trial and real-world data – preventing suboptimal data, costly delays, or additional follow-up visits which increase the burden to sites, patients, and sponsors. Using their myMedidata account, patients can easily be given the choice to opt-in for their RWD to be linked to clinical trial or registry data.
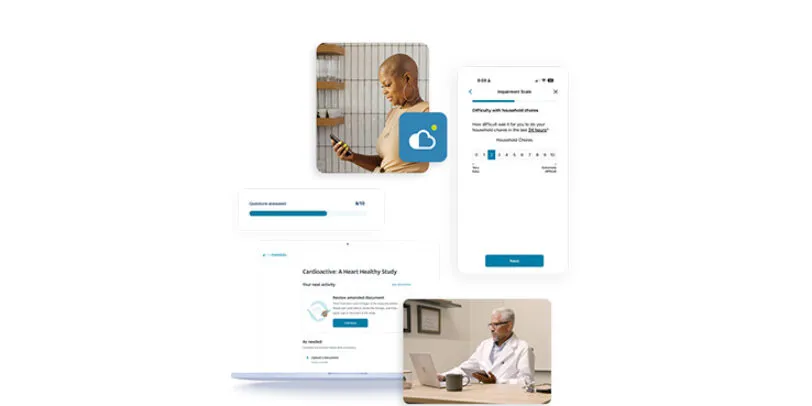
eCOA
eCOA provides a flexible, intuitive model for capturing patient data that is designed to make it easier for patients to engage in clinical trials. Built as part of the unified Medidata platform, eCOA improves your study experience with flexible deployment options, a groundbreaking global instrument library, and dedicated services and support.
Learn More

Enabling Clinical Trial Continuity with LIVE Video Visits
Hear a multi-perspective panel discussion on the challenges and opportunities of adopting a decentralized model for clinical trials and ensuring clinical trial continuity with live video visits.

Decentralized Clinical Trials: The Future of Clinical Research is Here
Download this white paper to read about the changing landscape around decentralized clinical trials, the types of solutions used to run DCTs, and how they benefit Patients, Sponsors, and Sites.

Site Perspectives on DCTs
In collaboration with SCRS, Medidata surveyed sites on their use of DCT solutions and technologies over the last two years. Read this whitepaper to see the results of that survey.