Rave EDC (Electronic Data Capture)
Medidata’s Rave EDC (Electronic Data Capture) is the most advanced, robust, and secure EDC system for clinical trial site, patient, and lab data capture and management.
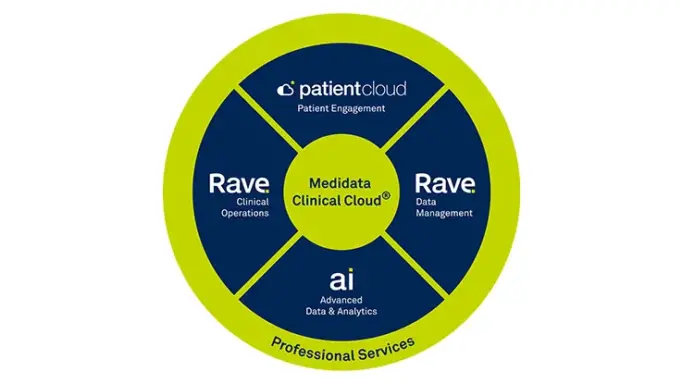
Rave EDC is the cornerstone of the Medidata Platform – the unified clinical research platform that connects processes, eliminates data reconciliation, and delivers cross-functional and cross-study data insights.
Why Choose the Rave EDC System?

Unparalleled Experience and Expertise in EDC
Medidata is the pioneer in Electronic Data Capture (EDC), trusted by you to run over 30,000 clinical trials, capturing data from millions of patients.
In the EDC Benchmarking and Market Dynamics report (June 2023, Industry Standard Research), Rave EDC was rated the most preferred, most recently used, and the leader among EDC providers.

Flexibility and Scalability for All Your Studies
Rave EDC has the flexibility and scalability to run all your biopharmaceutical or medical device studies, regardless of size, phase or therapeutic areas.
Our study design capabilities implement all protocol designs, no matter how simple or complex. And our infrastructure easily hosts your largest study populations and datasets.
Mid-Study Changes with No Downtime
Medidata and our customers perform an average of over 30,000 mid-study changes per year, including for studies with tens of thousands of patients. The combination of our technology and experienced Professional Services team ensures smooth implementation of mid-study changes, with no downtime, for even the most complex, large-scale, and time-critical trials.

Best in Class Data Security, Privacy, and Quality
Ensure the security and privacy of your critical clinical trial data and the privacy of your patients.
Medidata’s data security, privacy and quality processes, systems and certifications are second-to-none in the Life Sciences industry.
Accelerated Decision-Making for Clinical Operations
Rave EDC is unified with Clinical Operations capabilities on the Medidata Platform.
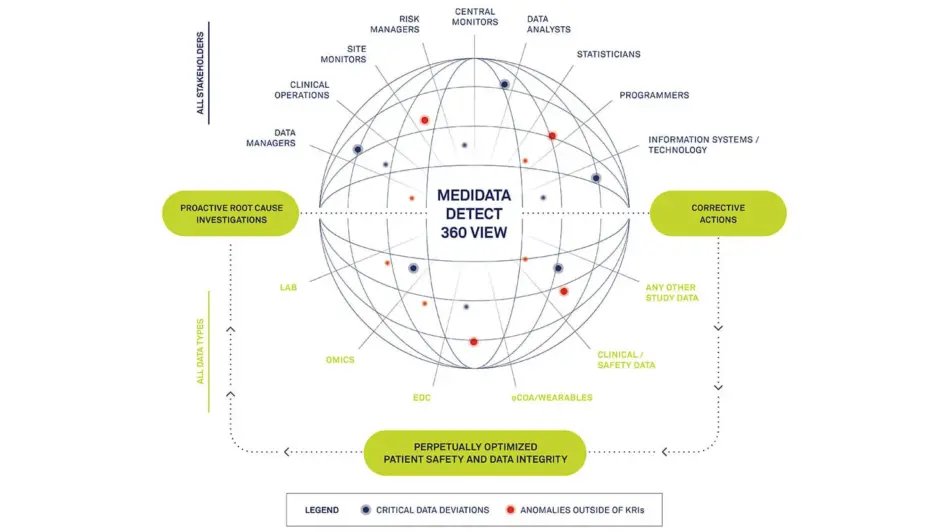
Through automatic data population from Rave EDC to Rave CTMS and Medidata Detect, your CRAs and study managers realize more efficient workflows and immediate data oversight. Detect compiles complete patient profiles and delivers automated, intelligent insights into data trends and anomalies to alert your data managers and central monitors to issues before they become systemic.

“The best qualities of Rave EDC are how user-friendly [it is] for the sites to enter data…, how the query management is very efficient, and [the ease of] getting the data out.”
– Vijay Chundru, Senior Director, EDC Programming Team, Global Clinical Data Operations, Jazz Pharmaceuticals

“We had a study with 6000 patients on it. [With Rave EDC] You could change 6000 patients in hours, whereas another platform took four months to adapt their CRF [Case Report Form] because the platform just wasn’t capable. I know when you say to people, ‘How long is that going to take to update the CRF?’……. ‘Once it’s all signed off, it’ll be an hour maximum,” Other platforms……definitely do take a lot longer.”
– Ian Howson, Senior Manager, Database Programming, Parexel
Contact an Expert
We would love to hear from you!
Want to learn more about our products and solutions or need a demo? Fill out this form to have a Sales representative reach out to you.
If you need technical support, please contact our Helpdesk.
Contact an Expert
We would love to hear from you!
Want to learn more about our products and solutions or need a demo? Fill out this form to have a Sales representative reach out to you.
If you need technical support, please contact our Helpdesk.
Key Features of Rave EDC
Make Data Entry Easier and Faster For Your Sites
Rave Companion reduces clinical trial data entry efforts for sites by making it simpler and faster to get source data from any system (e.g., EHR – electronic health record) or document (e.g., lab values in a spreadsheet) into Rave EDC.
Centralized Administration and Master Data
Centrally manage your users, roles, studies, and sites across all Rave EDC (and other products on the Medidata Platform) studies.
Eliminate study master data duplication and inconsistencies (e.g., different IDs for the same sites in different applications).
Give users a single place to login to all systems with the same username and password.
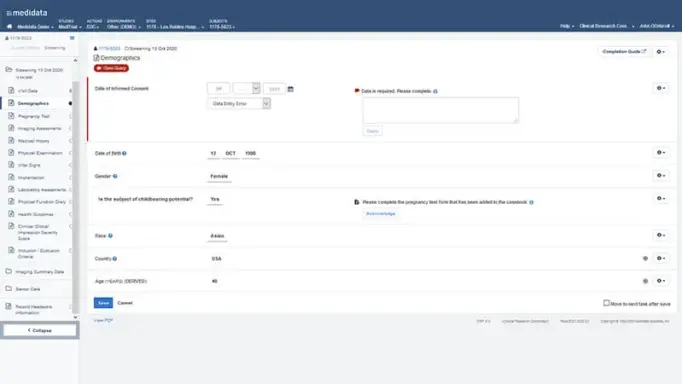
Real-time Data Validation
Make sure the right data is entered at the time of entry, not after the case report form is saved.
Improve your sites’ data entry efficiency and data quality with Rave EDC’s real-time edit checks.
Patient Data Surveillance
Medidata Patient Data Surveillance is the latest innovation to Rave EDC, combining two powerful data management solutions.The first solution is Patient Profiles which enables intuitive safety and medical reviews. The second solution is Data Reviewer, which aggregates patient data from almost any source for review, interrogation, and reconciliation, all from a single location.
Reporting and Analytics
Make informed decisions with real-time study insights through dashboards and standard/ad-hoc reports, and one-click access from reports to relevant forms.
Standard reports include study data monitoring, study admin, lab admin, and dictionary coding. Visualize your key study metrics such as enrollment tracking and data cleaning progress.
Ad-hoc reports use industry-standard software to let you perform a deeper analysis of your study data.

“The best qualities of Rave EDC are how user-friendly [it is] for the sites to enter data…, how the query management is very efficient, and [the ease of] getting the data out.”
– Vijay Chundru, Senior Director, EDC Programming Team, Global Clinical Data Operations, Jazz Pharmaceuticals

“This is where working with a partner like Medidata, that builds solutions such as Rave EDC and others, … helps us really leverage the extended expertise over years that they’ve built, to have reassurance and work on a robust platform.”
– Hassan Kadhim, Global Head of Clinical Trial Business Capabilities, BMS

“We had a study with 6000 patients on it. [With Rave EDC] You could change 6000 patients in hours, whereas another platform took four months to adapt their CRF [Case Report Form] because the platform just wasn’t capable. I know when you say to people, ‘How long is that going to take to update the CRF?’……. ‘Once it’s all signed off, it’ll be an hour maximum,” Other platforms……definitely do take a lot longer.”
– Ian Howson, Senior Manager, Database Programming, Parexel
Related Solutions

Rave Data Management
Rave EDC is at the heart of Medidata’s unified solution for Clinical Data Management, enabling aggregation and reconciliation of data from multiple sources – Medidata eConsent, Medidata eCOA, MyMedidata, Rave RTSM, Rave Imaging and Sensor Cloud; and intelligent data review and analysis with Rave TSDV and Medidata Detect.

Rave Coder – Medical Coding for Clinical Trials
Rave Coder equips your studies with fast, accurate medical coding for verbatim terms from Rave EDC and external sources using Machine Learning and Natural Language Processing.
Your coding workflow is streamlined through integration with Rave EDC.

Rave Safety Gateway – Safety Data Transmission
Rave Safety Gateway delivers precise, accurate, and efficient transmission of AEs and SAEs in Rave EDC to your safety system.
Eliminate duplicate data entry, accelerate the transmission of safety case data and reduce data reconciliation between clinical and safety teams.
Learn More

Modernizing Clinical Data Management to Be Scalable, Flexible, and Intelligent
This white paper provides a summary of why Clinical Data Management (CDM) must quickly adapt to the mounting data pressures in modern clinical trials and discusses the three pillars that form the foundation of a modern intelligent CDM platform that is needed to succeed in an increasingly complex clinical trial world.

TissueTech: Launching a Phase 3 Pivotal Trial in a Pandemic with the Medidata Platform
Learn how during the COVID-19 pandemic, the Medidata Platform equipped TissueTech to immediately pivot to a remote and centralized monitoring model.

The Next-Generation of Clinical Data Management
The clinical data landscape continues to evolve, but the way data is managed (reviewed and cleaned) has not kept pace with the growth in data sources, types, volume, and velocity. This infographic explores the challenges and proposes a solution to modernize clinical data capture and management.