

Innovation Central: Eli Lilly
Welcome to Innovation Central. Here, you’ll find a curated selection of our latest solutions, best practices, thought leadership, and practical insights to help you optimize your clinical trials. Come explore, ideate, and innovate.
Bookmark this page and visit frequently to stay up-to-date with all the latest.
Solutions & Services
Take a closer look at the solutions and services BioMarin currently uses to help bring about better patient outcomes.

Medidata Rave
Medidata Rave is a cloud-based platform used in clinical trials for managing and capturing data. It’s a system for electronic data capture (EDC) that helps organizations streamline the clinical trial process, improve data quality, and accelerate research. It allows users to enter and manage patient data, including lab results, adverse events, and other relevant information, through customized forms.
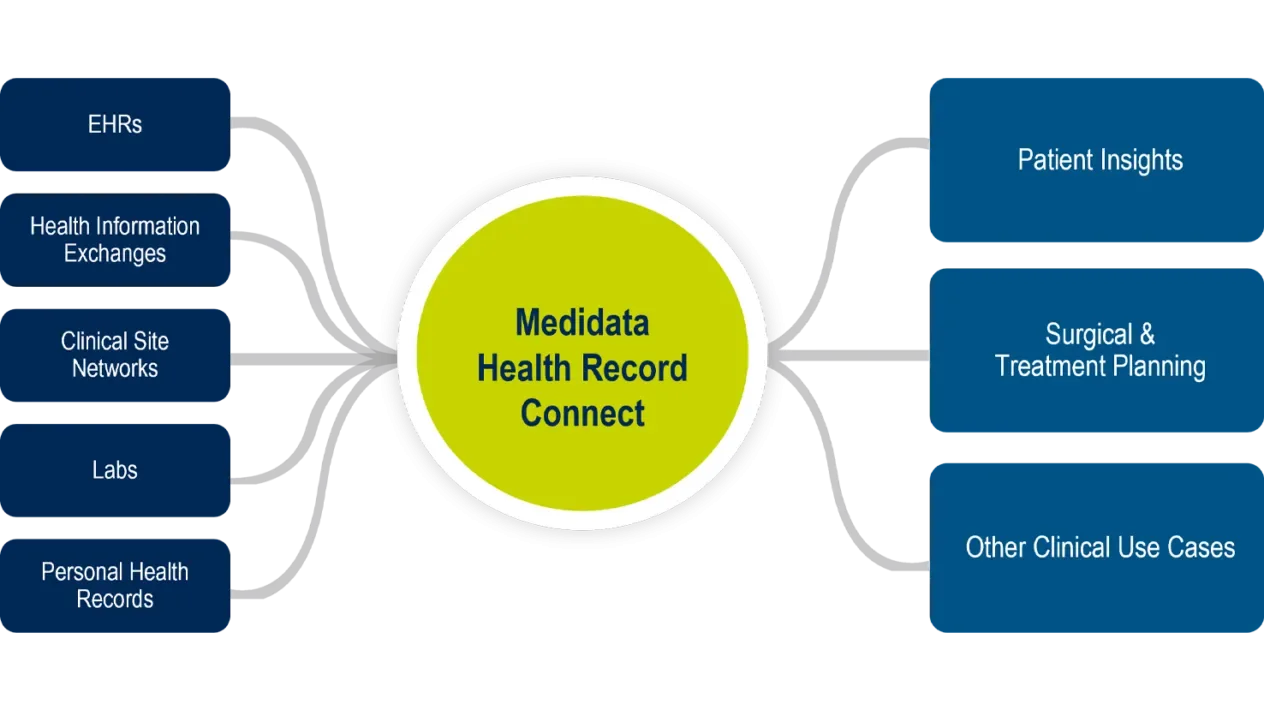
Health Record Connect
Medidata Health Record Connect is an EHR (electronic health record) integration and interoperability engine for securely and compliantly acquiring, transforming, and exchanging EHR data. It powers a whole new level of collaboration and visibility into a patient’s critical healthcare information.

Clinical Data Studio
Medidata Clinical Data Studio is a data management and quality experience that provides seamless access to integrated data from Medidata and non-Medidata sources. It leverages AI to streamline data aggregation, standardization, and management workflows so that multiple users can review real-time data to shorten timelines, reduce risk, and ensure safety.

RTSM
RTSM is built on Rave EDC, so there is no double data entry and minimal reconciliation expediting study start-up and study-close out. Rave RTSM streamlines your operations and provides real-time visibility for your study teams.
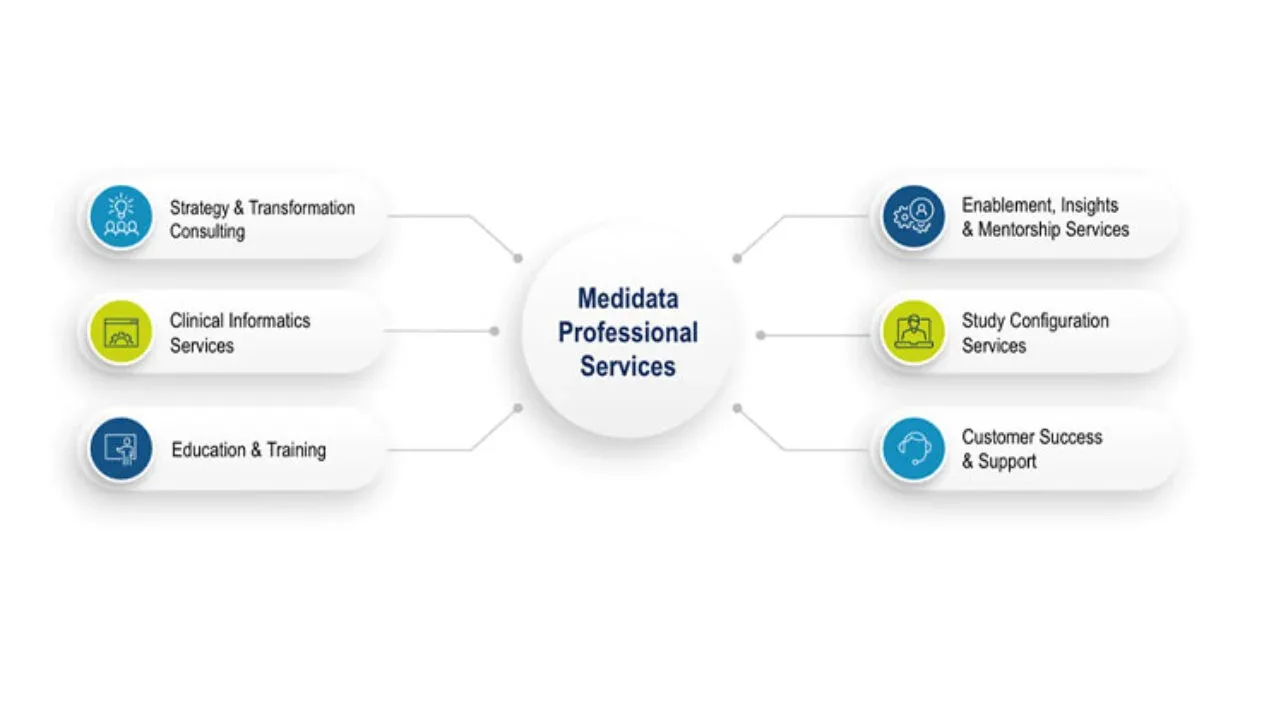
Professional Services
Medidata has paved the way for the next generation of clinical trials including specialized solutions like decentralized clinical trials, patient-centric solutions, synthetic control arm studies, and advanced analytics.
Patient Cloud
Patient Cloud is a suite of powerful solutions that makes it simple and engaging for patients to participate in any clinical trial – so your trials are easier, faster, and produce better results. Built into the Medidata Platform, Patient Cloud solutions combine Medidata’s leading clinical trial technology with unmatched patient centricity by design.
eCOA (Clinical Outcome Assessment) is revolutionizing the way sponsors, CROs, and sites collect electronic data from patients, physicians, and caregivers. Medidata’s eCOA capability is built using Designer, enabling Sponsors and CRO partners to build rich patient experiences via intuitive drag and drop screen templates and visual workflow tools. Available through myMedidata, patients can easily access their study tasks through the web or an app on any mobile device.

Medidata’s Consent is an innovative, regulatory-compliant, patient-friendly, electronic consent system for clinical trials. Whether onsite or remote, Medidata Consent automates the patient enrollment process and onboards patients directly into Rave EDC, improving overall consent tracking management, reducing informed consent errors, and easing the administrative burden for sites and study teams.

Medidata Sensor Cloud supports over 50 validated measures and algorithms across episodic and continuous sensors. We equip patients with a simple integrated experience to collect objective sensor data alongside subjective eCOA data. Clinicians and study managers have access to powerful study management and reporting tools to help monitor patient compliance and see meaningful insights and trends. Our data science experts deliver advanced analytics that combine objective and subjective data for complete patient views.
Patient engagement shouldn’t end with a patient’s last visit. With myMedidata Registries, introduce post-trial patient engagement in your clinical trials and enable long-term follow-up, patient data return, enhanced safety monitoring, and gathering of new clinical insights.

Medidata’s Patient Insights program infuses the patient perspective into the software development life cycle to create technical solutions that improve the overall patient experience in clinical research operations.
Imaging & Adjudicate
Learn more about Medidata’s Imaging and Adjudicate offerings.
Medidata AI
Medidata’s unique ability to enhance clinical development through AI stems from our extensive industry expertise. With data from over 36K clinical trials and 11M patients, we offer unparalleled insights that inform every stage of the clinical trial process.
Intelligent Trials
Design smarter studies and drive operational performance with Medidata’s industry-wide, real-time, and historical trial data combined with AI-powered analytics. Confidently optimize your protocol to balance scientific goals with operational feasibility, select high-performing sites that support enrollment and diversity targets, and monitor progress to proactively mitigate risks.
Medidata Link
Medidata Link enables clinical trial data linkage with real-world data (RWD) at the patient level to enhance evidence generation beyond what is possible in traditional clinical trials. By creating an enriched view of the patient journey before, during, and after your trial’s completion, Medidata Link generates insights that enable you to accelerate patients’ access to innovative treatments while minimizing site burden and costs.

Synthetic Control Arm
Medidata AI Synthetic Control Arm (SCA®) offers the only external control arm created with cross-industry historical clinical trial data from 30,000 clinical trials and 9 million patients.
Trial Design
Our team of industry experts partners with you to leverage this data to accelerate medical breakthroughs by delivering meaningful insights to create safer, effective clinical trial designs. We also offer Simulants, a pioneering synthetic data tool that delivers the insights contained within historical clinical trial data while safeguarding patient privacy and intellectual property.
News, Resources and Thought Leadership

Stay informed with the latest news, updates, and insights from Medidata, covering innovations, announcements, and industry developments.

Medidata unveils transformative solutions and collaborations at NEXT New York, driving paradigm shift in life sciences and healthcare.

Explore how Medidata is making headlines around the world with the latest media coverage, press features, and industry recognition.

Browse our archive of past webinars featuring expert discussions and actionable insights.

Enhance business agility with dynamic planning that integrates strategy, operations, and finance for rapid adaption to market shifts.
Upcoming Events
Meet Your Account Team








