Innovation Central: Bristol Myers Squibb
Welcome to Innovation Central for Bristol Myers Squibb. Here, you’ll find a curated selection of our latest solutions, best practices, thought leadership, and practical insights to help you optimize your clinical trials. Come explore, ideate, and innovate.
Bookmark this page and visit frequently to stay up-to-date with all the latest.
Medidata Solutions and Services

Unlock the True Power of Clinical Research Data
Medidata Clinical Data Studio is a data management and quality experience that provides seamless access to integrated data from Medidata and non-Medidata sources. It leverages AI to streamline data aggregation, standardization, and management workflows so that multiple users can review real-time data to shorten timelines, reduce risk, and ensure safety.
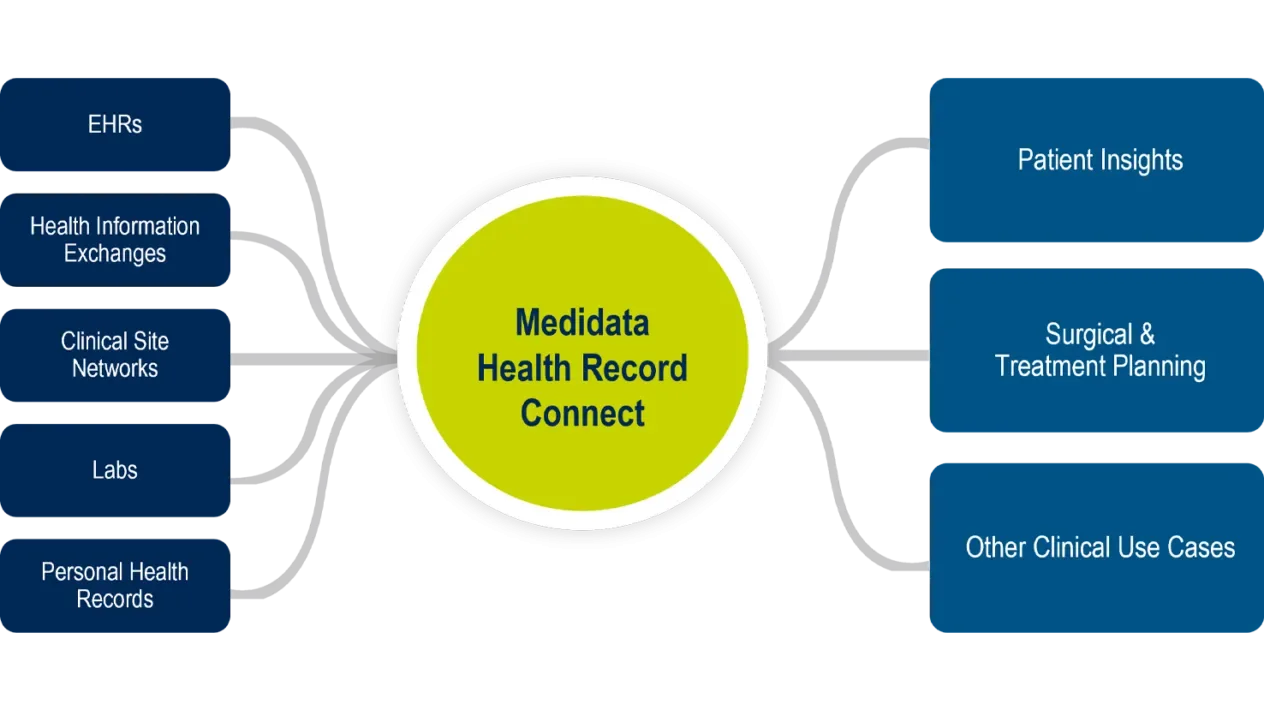
Health Record Connect
Medidata Health Record Connect is an EHR (electronic health record) integration and interoperability engine for securely and compliantly acquiring, transforming, and exchanging EHR data. It powers a whole new level of collaboration and visibility into a patient’s critical healthcare information. Leveraging a multi-pronged approach to connectivity, Medidata Health Record Connect ensures that the right data from the right site and patient can be securely acquired, transformed, and presented to the appropriate users in a single user experience.
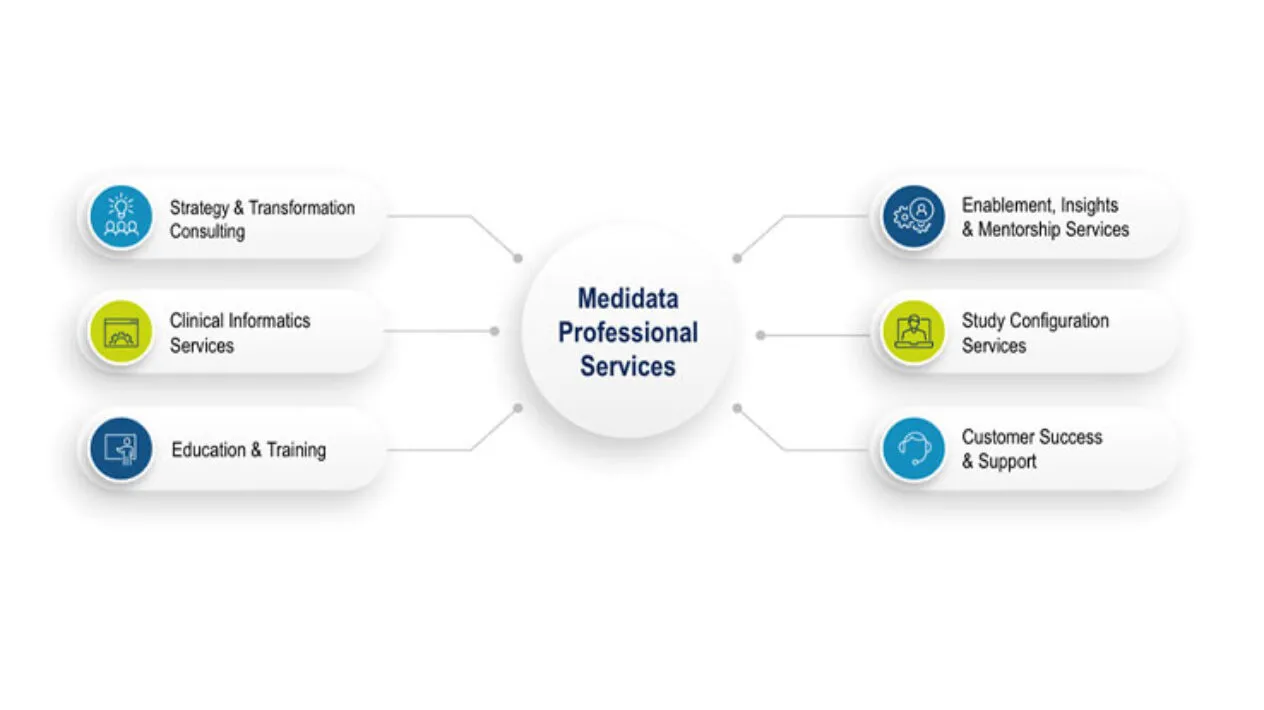
Clinical Informatics Services
One of the latest service offerings from Clinical Informatics Services is called Interim Lock, which allows users to take a data cut of a database at any point in time while the study is in progress. This function temporarily locks the database for interim analyses and automates a previously very manual, labor-intensive process.

Rave RTSM
Rave RTSM is the only fully pre-validated randomization and trial supply management solution that can be configured in minutes and enables mid-study changes with minimal downtime and change orders.
Sensors
Medidata Sensor Cloud supports over 50 validated measures and algorithms across episodic and continuous sensors. We equip patients with a simple integrated experience to collect objective sensor data alongside subjective eCOA data.

Medidata Rave
Medidata Rave is a cloud-based platform used in clinical trials for managing and capturing data. It’s a system for electronic data capture (EDC) that helps organizations streamline the clinical trial process, improve data quality, and accelerate research. It allows users to enter and manage patient data, including lab results, adverse events, and other relevant information, through customized forms.
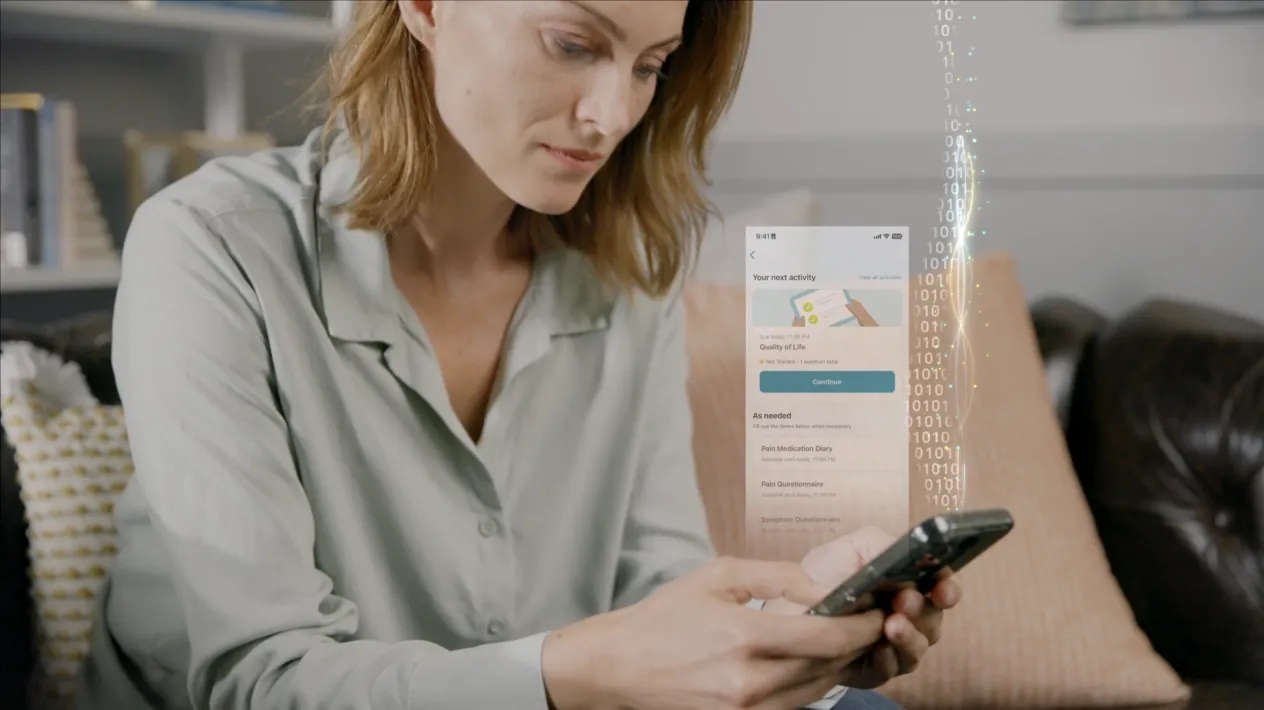
Upgrade Your Patients’ Clinical Trial Experiences with eCOA
eCOA (Clinical Outcome Assessment) is revolutionizing the way sponsors, CROs, and sites collect electronic data from patients, physicians, and caregivers. Medidata’s eCOA capability is built using Designer, enabling Sponsors and CRO partners to build rich patient experiences via intuitive drag and drop screen templates and visual workflow tools. Available through myMedidata, patients can easily access their study tasks through the web or an app on any mobile device.

AI Everywhere
Medidata’s unique ability to enhance clinical development through AI stems from our extensive industry expertise. With data from over 36K clinical trials and 11M patients, we offer unparalleled insights that inform every stage of the clinical trial process. Our AI-driven solutions seamlessly integrate into the Medidata Platform, enabling efficient data management, predictive modeling, and operational optimization. This comprehensive approach ensures that our AI tools are powerful and precisely tailored to the complexities of clinical research, setting us apart in the industry.

Rave Imaging | Clinical Trial Imaging
More than 50 percent of trials involve medical imaging. Manage these studies faster, cheaper, and with lower risk when you choose Rave Imaging. Rave Imaging provides cloud-based, secure management for all your medical imaging tasks in an innovative and intuitive system. Gain visibility and full control over your imaging data while simplifying processes for sites, sponsors, and core labs alike.

Empowering Patients Right From the Start
Medidata’s eConsent is an innovative, regulatory-compliant, patient-friendly, electronic consent system for clinical trials. Whether onsite or remote, Medidata eConsent automates the patient enrollment process and onboards patients directly into Rave EDC, improving overall consent tracking management, reducing informed consent errors, and easing the administrative burden for sites and study teams. It also enhances the patient experience with easy-to-understand clinical trial information while improving patient compliance and boosting patient engagement.
Value Realization through Medidata Expertise and Innovation
Medidata has paved the way for the next generation of clinical trials including specialized solutions like decentralized clinical trials, patient-centric solutions, synthetic control arm studies, and advanced analytics.
We provide comprehensive professional services that support the entire clinical trial process, from pre-planning to post-go-live.
BIOVIA Solutions & Services

Improve Lab Productivity
Simplify, streamline, and strengthen your lab workflows with BIOVIA ONE Lab—a unified laboratory informatics solution that supports all stages of a therapeutic’s lab lifecycle. ONE Lab is the only solution that is flexible and configurable enough to be used in drug research labs, drug process development, and quality control labs. Take your organization’s productivity to the next level by digitalizing every step of the process, speeding time to market.

Accelerate Drug Discovery and Development
In silico software for drug discovery and development help streamline pharmaceutical R&D. Researchers can design and optimize drug candidates virtually before moving on to expensive lab testing, saving countless hours of research and thousands of dollars in R&D costs. Incorporation of these tools into existing workflows is crucial for modern drug discovery and development projects.

Quality Management Software for BioPharma
Quality is essential for patient safety, treatment efficacy, sustainability and protection of brand reputation. We help organizations achieve quality and business excellence with a new and comprehensive data-centric approach — all based on digital continuity, data integrity and a ‘single source of truth’.
News, Resources and Thought Leadership

Stay informed with the latest news, updates, and insights from Medidata, covering innovations, announcements, and industry developments.

Medidata unveils transformative solutions and collaborations at NEXT New York, driving paradigm shift in life sciences and healthcare.

Explore how Medidata is making headlines around the world with the latest media coverage, press features, and industry recognition.

Browse our archive of past webinars featuring expert discussions and actionable insights.

Explore expert insights and essential resources all in one place.

Email us at the link below to find out about upcoming Roadmap Reviews
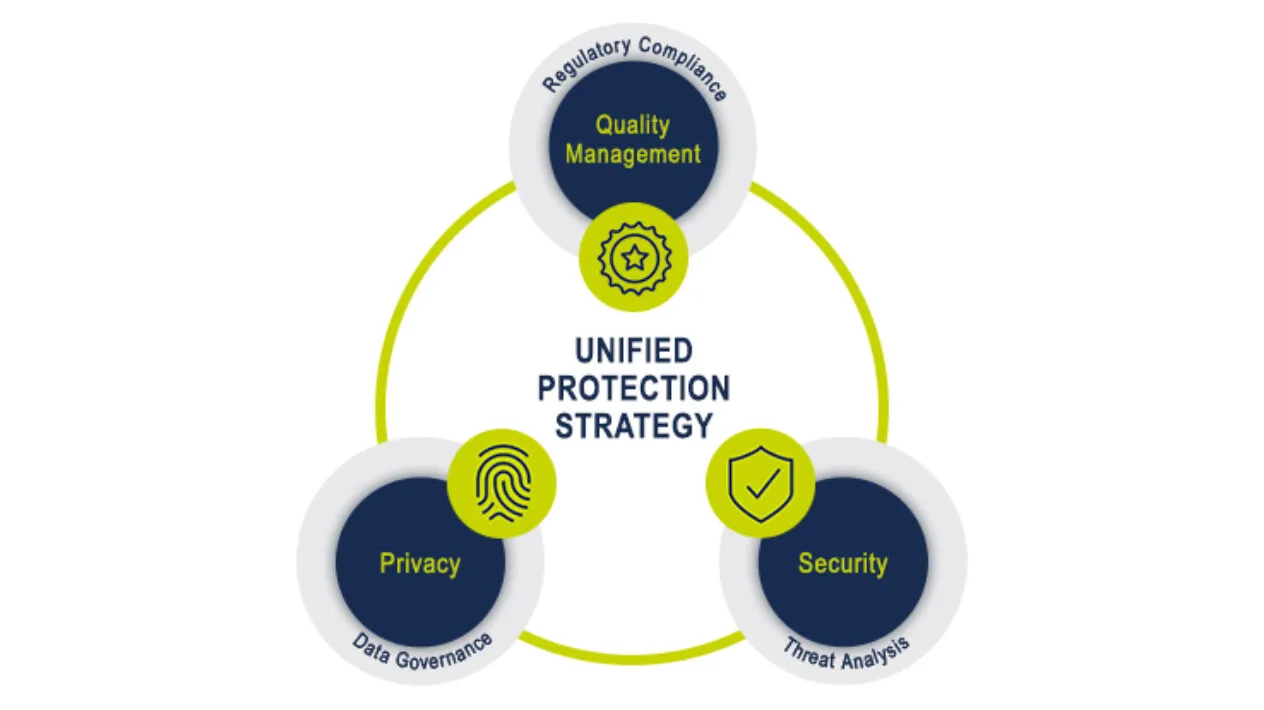
Audit & Regulatory Requirements
Leading With Trust and Transparency
Medidata’s Unified Protection Strategy encompasses our secure, stable, and scalable cloud platform, robust data governance processes, and an inspection-ready quality management system – all critical enablers to successful clinical trial execution. Our Information Security, Privacy, and Quality Management teams work in unison to safeguard your data and provide solutions that ensure your regulatory compliance.