How to Take Your Imaging Trials to the Next Level with Automation and AI
The use of medical imaging in clinical research is becoming more common, with a growing number of indications over the past few years. Yet, managing medical images is complex, leading to significant quality challenges and trial delays if not performed correctly.
Below is a depiction of the typical lifecycle of a medical imaging trial. The interconnected nature of these trials means that each step of the process must be flawless. Otherwise, downstream steps will be negatively impacted, which can quickly snowball.
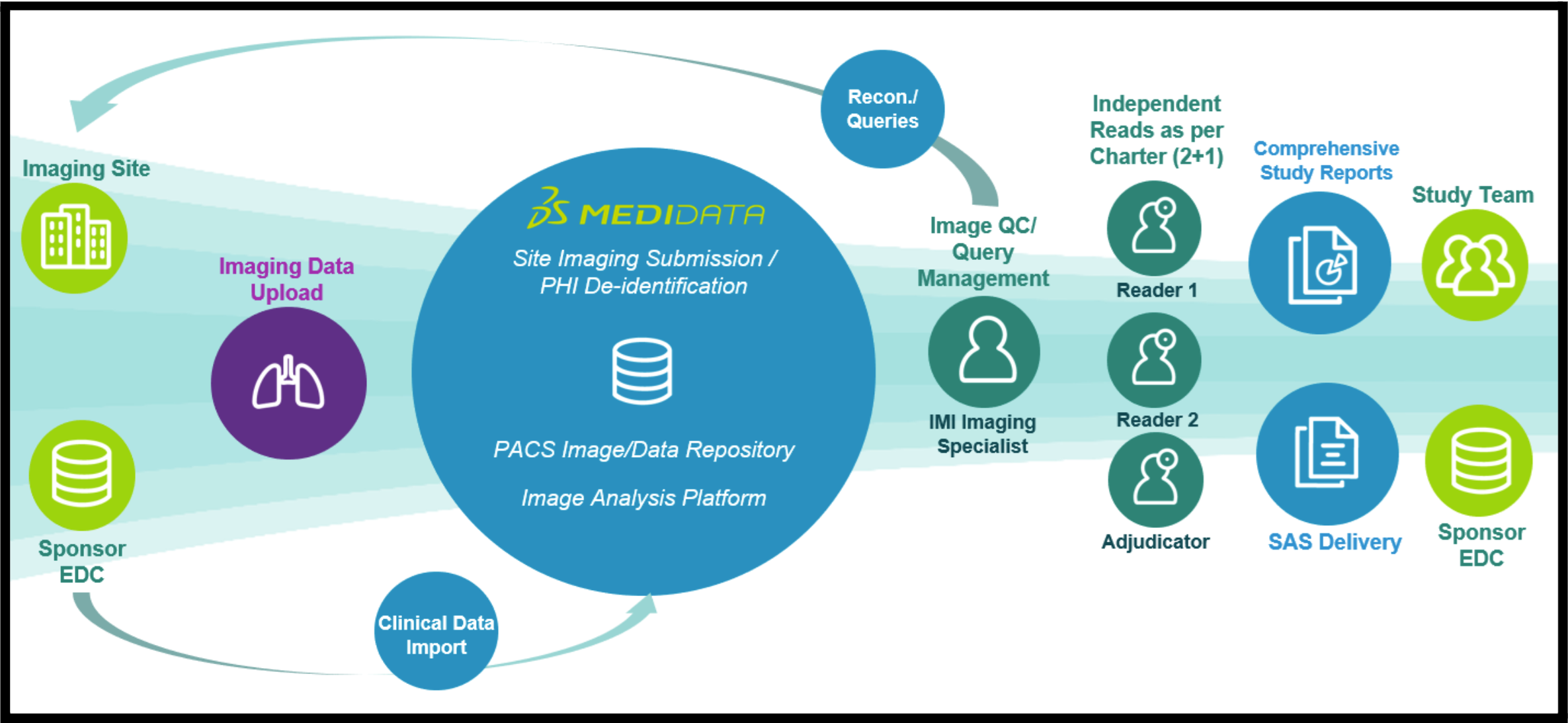
Figure 1: Sample Medical Imaging Trial Lifecycle.
Experts from ICON Medical Imaging and Medidata recently delivered a webinar that discussed how sponsors can leverage automation and AI in their medical imaging trials to provide the best opportunity for success. Some of the ways that automation can help include:
- Reducing the number of manual steps
- Creating efficiencies that accelerate trial timelines
- Generating high-quality medical imaging data that is “submission-ready”
Below is a summary of the webinar, mainly focusing on the common challenges associated with imaging trials and how to circumvent them via automation.
Key Challenges in Image Trials
- Inefficient and poor-quality image uploads and submissions
- Multiple data sources and lack of standardized data across patients, sites, and trials
- Manual data reconciliation, which is burdensome for sites and data managers
- Different requirements than site standard of care
- Multiple sources of potential Protected Health Information (PHI)
How Automation Enhances Clinical Trial Imaging
Image automation has proven highly effective for streamlining and simplifying medical image data collection and management to circumvent key challenges.
Integrating EDC and Imaging Platforms Is Key
Automated processes are effective when EDC and imaging platforms are integrated (e.g., Medidata's Rave EDC and Rave Imaging). This is because end-to-end processes will be fully integrated, allowing users real-time visibility from the EDC. Ideally, the integration should be two-way (critical data flow automatically from the EDC to the imaging platform and vice versa). This enhances data quality while reducing the burden on sites and data managers, which do not have to enter the same data multiple times.
A fully integrated system also aids in setting up a robust reconciliation process to make sure that central labs receive and review all relevant images acquired by a site. This critical process increases the likelihood that all endpoint assessments are conducted on time.
Automated Processes Can Shrink Trial Timelines
Medical image management system automation also has a direct impact on trial timelines. Trial start-up efficiencies are achieved via templates and platform features that let users replicate trial builds rather than having to reconfigure them de novo. Startup timelines have been condensed to as little as three weeks from the typical 8–11-week time frame when these features are leveraged.
Edit Checks for Protocol Compliance
Automated tools and procedures also provide edit checks for compliance with acquisition protocols, such as verifying information stored in DICOM® (Digital Imaging and Communications in Medicine) tags (e.g., slice thickness, imaging sequence) or other formats, such as non-DICOM data and laboratory data. These edit checks flag deviations as early as possible to reduce the likelihood of unevaluable or duplicate images, etc.
Automated PHI identification and removal (e.g., pixel PHI) and methods to filter and remove irrelevant images can enhance data quality and reduce site burden. Artificial intelligence (AI) algorithms that automatically detect text in CT and MR images and perform bulk redactions can save even more time when dealing with pixel PHI.
The future of imaging trials has arrived, and further innovations will include more automation. Additional AI applications will soon be available from Medidata, including the following:
- Subject verification algorithm that alerts users if uploads do not belong to the same patient
- Lesion detection algorithm to support solid tumor trial reviews that provides automated lesion detection and measurements, including volumetric measurements
- Image library for storing images and analytical data, allowing them to be readily available for future access and additional analysis
Watch our webinar to learn more:
Explore Related Articles
Contact Us


