Medidata CTMS
Simple to Integrate. Simple to Use. Simple to Scale.
Medidata CTMS, part of the Medidata Study Experience, optimizes clinical trial management by centralizing clinical and operational data, streamlining activities, and provides continuous oversight.
Benefits of Medidata CTMS

Easy to Adopt
A straightforward user experience matches the way your teams work reducing user training and accelerating time to adoption. Automated workflows streamline manual processes for key activities, and dashboards focus you on what matters most. Milestones, tasks, issues, and document status are tracked in one place. Data is entered once and used everywhere, eliminating information silos, providing tremendous efficiency for site and monitoring staff.
Scalable and Reliable
Built on a cloud-based, single-instance, multi-tenant architecture, Medidata CTMS is designed for scale and reliability, ensuring optimal performance and future growth. Medidata CTMS scales from early phase through late phase trials. It also integrates with your existing technology and workflows.
Reduce Avoidable Startup Delay
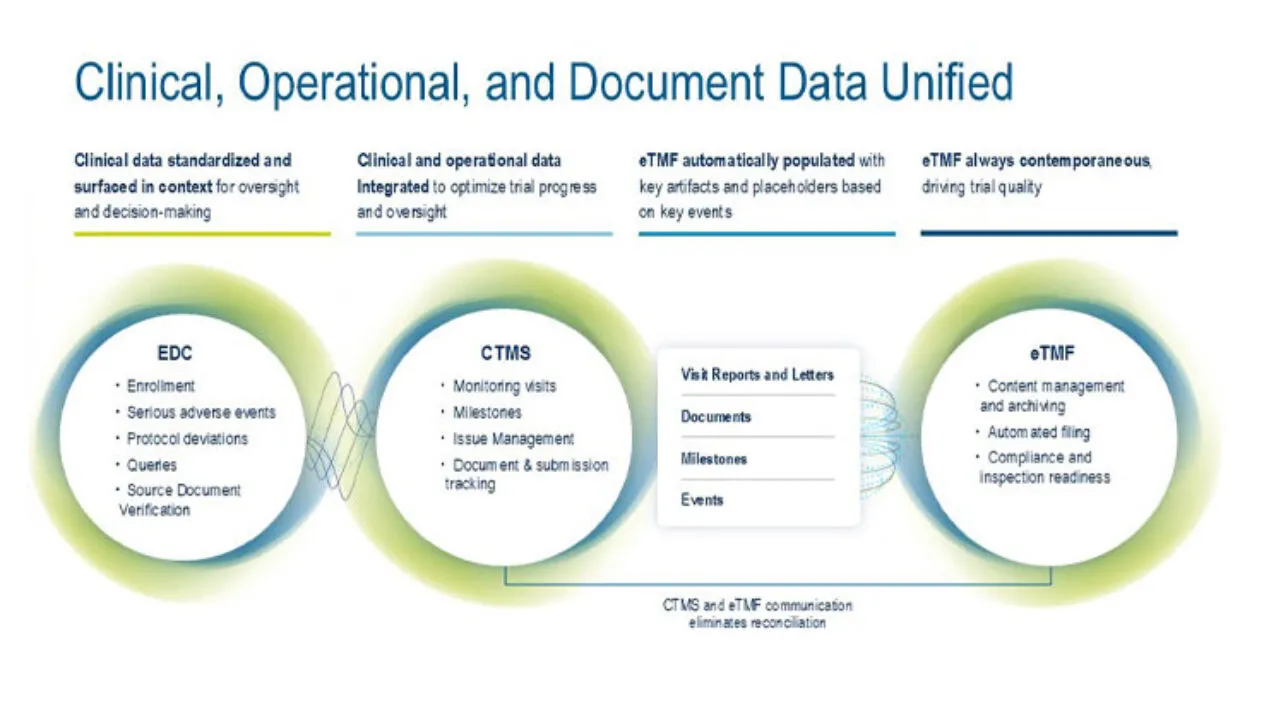
Study startup activities are embedded within Medidata CTMS and Medidata eTMF, where site-specific required milestones and tasks are tracked in one place. A closed-loop system of site activation activities and issue management eliminates multiple status spreadsheets and tracking reports, saving you time and effort.
Maximize Efficiency
Centralized configuration groups and templates offer flexibility across studies while ensuring standardization. Data from multiple sources is delivered directly to drive informed decisions.
White Glove Partner Ensures Success
Medidata’s Professional Services experts deliver comprehensive enablement that goes beyond implementation to maximize the system’s value.
Features of Medidata CTMS
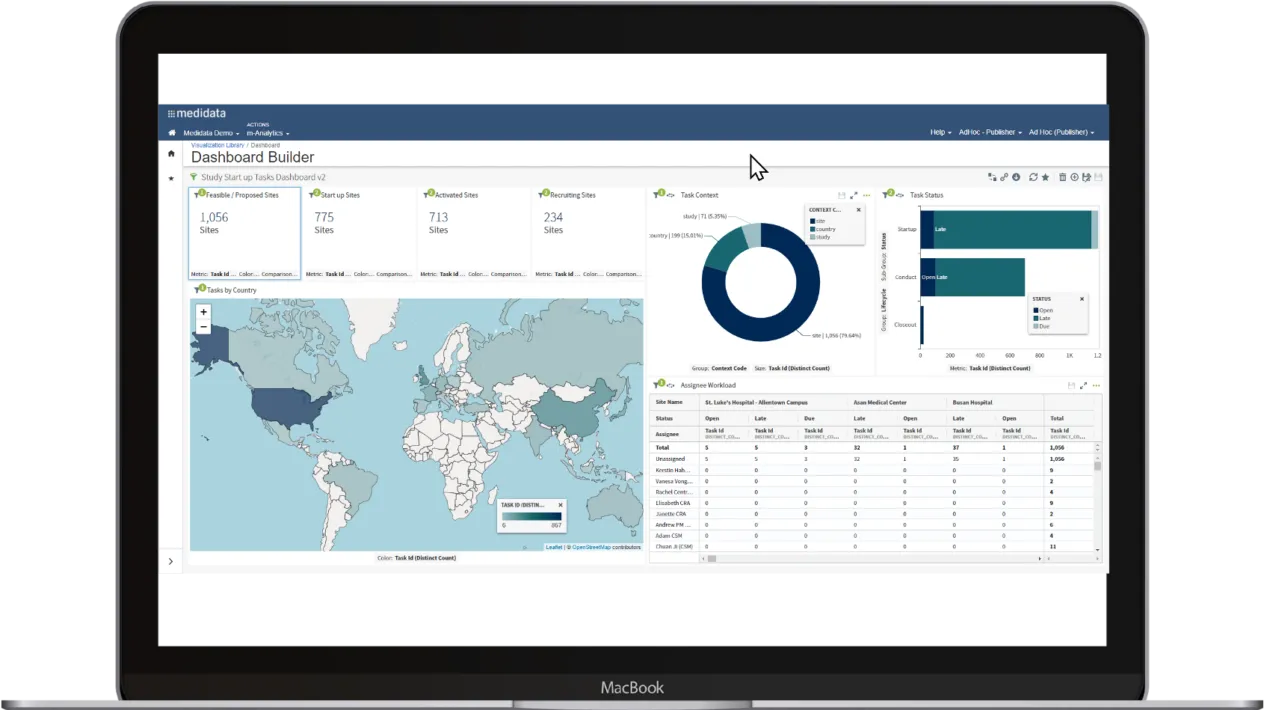
Powerful, Visual Analytics
Efficiently manage the entire study with data automatically populated from any EDC for dashboards, forecasts, and analysis. With Medidata CTMS Visual Analytics, create intuitive visuals by combining Medidata CTMS data across studies into a single visualization, report, or dashboard.
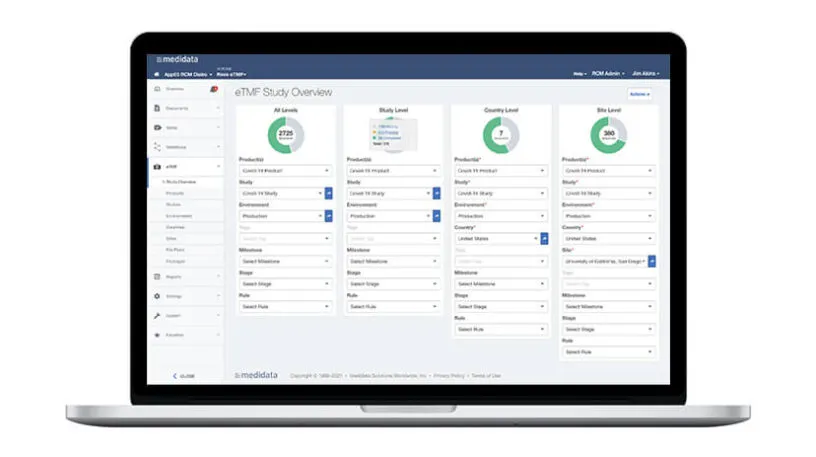
CTMS is tightly integrated with Rave EDC and Medidata eTMF to optimize collaboration with sites. Users upload documents once and the eTMF is automatically updated with data and content. Data is auto populated into monitoring visit reports, which are then automatically filed to the TMF. Track and manage required documents, submissions, and metadata at the study, country, and site levels across all stages. Templates and document packages simplify milestone and key event tracking, and integration with eTMF ensures data remains contemporaneous across applications.
Track clinical trial progress using configurable milestones that ensure timeline adherence and preserve trial integrity. Enrollment metrics at the study, country, and site levels provide actionable insights into critical events like underperforming sites or over-enrolling countries, optimizing resource planning and protecting trial timelines.

Easily move to a paradigm where interactions with sites are purpose-driven and with intention, focused on critical data and processes.
A modern, configurable site monitoring workspace provides CRAs with in-context data feeds to streamline preparation, execution, and follow-up activities. Reports and letters are automatically filed to the TMF, and issues can be created from anywhere within the workspace.
Related Solutions
Learn More

One Unified Hub for Digital Oversight of Your Trial
Medidata CTMS, unified on the Medidata Platform, is your transactional hub to natively and intelligently connect workflows, deliver data-driven insights, and foster collaboration – all powered by unified data capture.

Medidata CTMS Visual Analytics Fact Sheet
See how Visual Analytics for Medidata CTMS can drive efficient workflows, enable collaboration across teams and gain oversight of your study progress. With powerful visualizations and easy-to-use tools, you can take back control of your study.

Automated Clinical Trial Monitoring Workflows Make a Lean Team More Efficient
Learn how fast-growing biopharmaceutical company Enterin implemented Medidata CTMS to enable their resource-constrained team to work more effectively.

Fast Data Insights Improved Productivity and Client Centricity
Learn why and how Catalyst Clinical Research personnel identified a need for a scalable CTMS solution that would grow with their organization and support their customer throughout the drug development process, and why they selected Medidata CTMS.