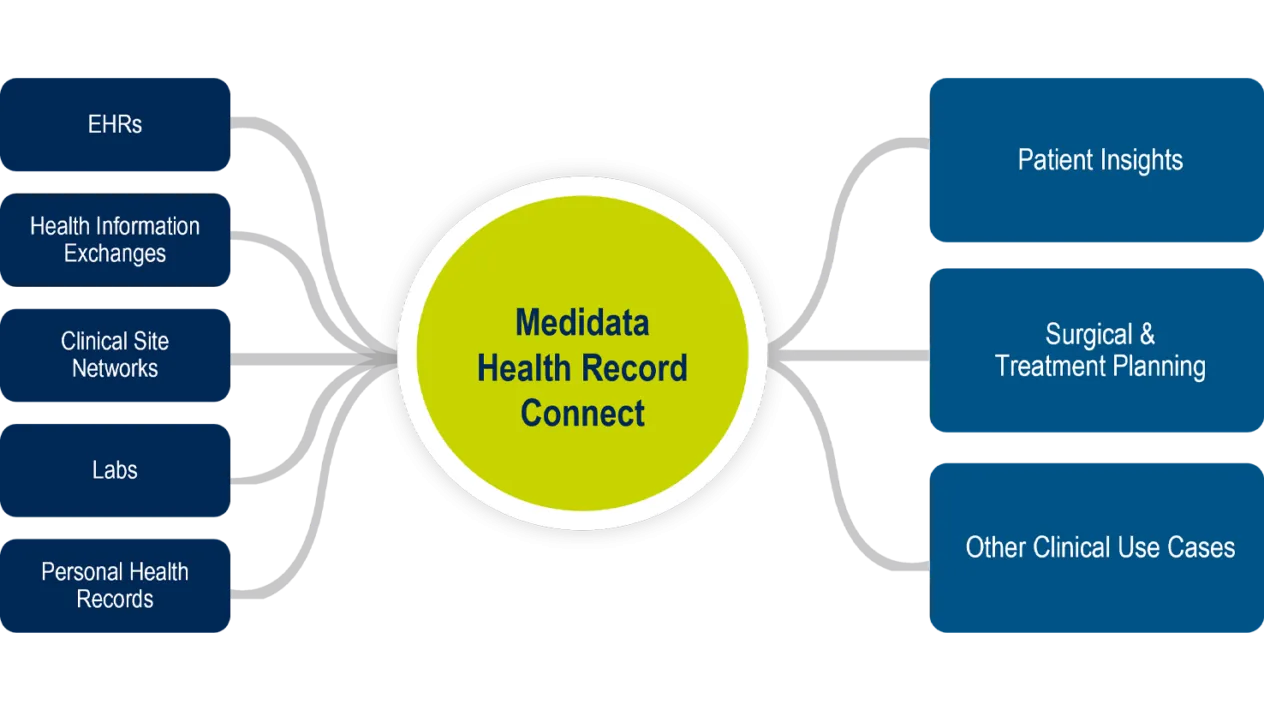
EHR Integration – Medidata Health Record Connect
Medidata Health Record Connect is an EHR (electronic health record) integration and interoperability engine for securely and compliantly acquiring, transforming, and exchanging EHR data. It powers a whole new level of collaboration and visibility into a patient’s critical healthcare information. Leveraging a multi-pronged approach to connectivity, Medidata Health Record Connect ensures that the right data from the right site and patient can be securely acquired, transformed, and presented to the appropriate users in a single user experience.
EHR to EDC Myth Busters
See how the myths are busted in under 90 seconds
Key Features and Benefits of Medidata Health Record Connect
Clinical trials are necessary treatment/care options (known as Clinical Research as a Care Option or CRAACO) for many patients. In some cases, interventional clinical trials are the only remaining treatment option for a patient. As the lines between lifelong healthcare data and the subset of the data required in a trial continue to blur, we must get smarter and more effective at engaging patients long-term and ensuring their care teams have the most complete data to inform their clinical decision-making. The capacity to manage a greater variety of complex data from a broader range of sources is no longer optional—it’s imperative. This is more than a vision — it’s a commitment to patients that we will advance research and healthcare as quickly and safely as possible by using every part of the data ecosystem available to us.
Medidata Health Record Connect works with Rave Companion to present matching EHR data to clinical trial sites as they complete Rave EDC forms. This dramatically speeds up data entry where the clinical trial forms require data that already exists in the patient’s healthcare record while ensuring that the site always has a complete set of information to inform their clinical decisions. The site’s eyes are always on the data, and nothing is submitted to Rave EDC until the site saves the form.
Connects to any healthcare system, using whatever protocol or standard is appropriate for each site, enabling quicker startup times and higher adoption rates.
Out-of-the-box scalability with connectivity to a wide range of healthcare organizations and physician practices through Health Information Exchanges (HIEs). No need to create one connection at a time.
Works with any format or protocol, including those commonly used to communicate healthcare information across the USA.
Leverage site healthcare data across all Medidata applications, regardless of communication format.
By pulling in data from multiple sources, users get a full view of the patient’s history, not just information from their own site, enabling richer patient insights.
Medidata Health Record Connect powers a wide range of workflows and use cases including, but not limited to, Medidata Surgical Planning, Real-World Data (RWD) aggregation and insights, and data collaboration within a clinical trial* using Rave Companion.
*Note: For interventional trials only. All users must be employed by the institution in which the data is accessed, under the supervision of a physician or PI (Principal Investigator) at all times, and be involved in the monitoring of safety events and study outcomes for patients enrolled in research.
Learn More
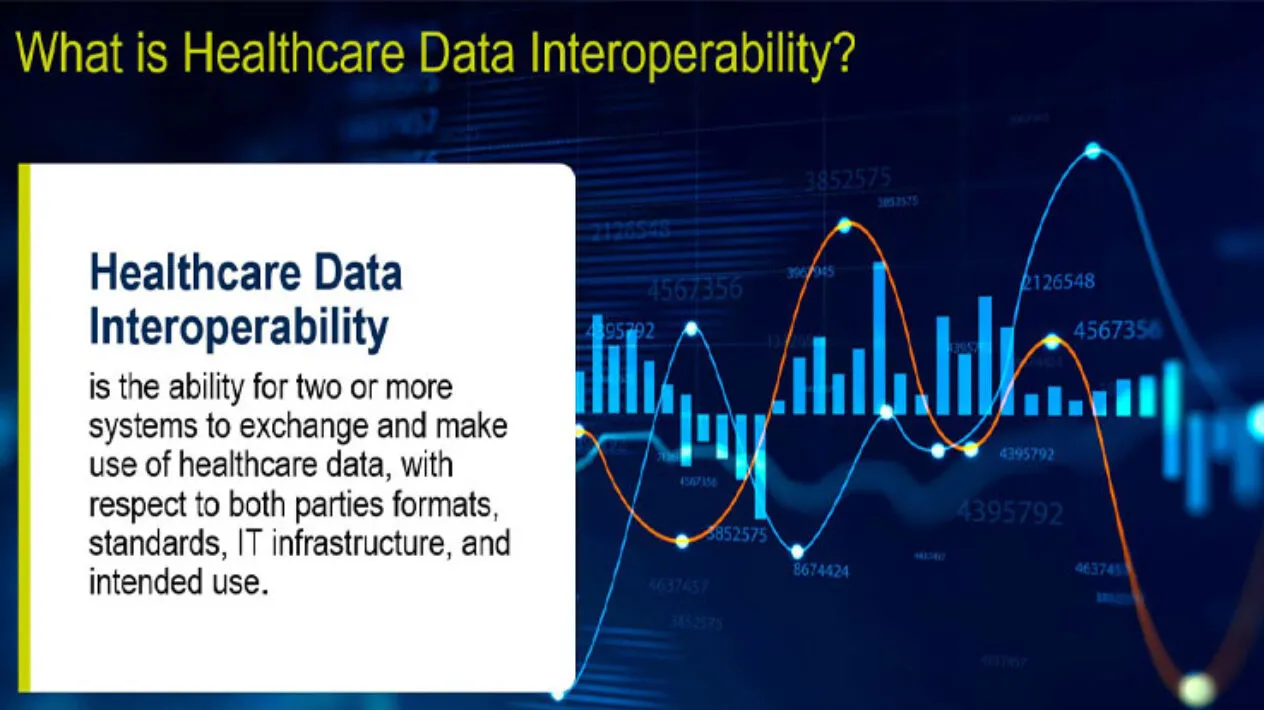
Bridging the Gap – TEFCA, Healthcare Data Interoperability, and Clinical Research Use Cases
The issues of healthcare data interoperability and integration have directly affected the ability to deliver better healthcare, significantly impacted patient safety, and hindered access to clear, complete source data and insights for analysis and research. This, in turn, has had a direct impact on clinical trials. These issues have plagued the industry for decades. So, it’s no surprise that key questions asked are: why is this still an issue, and what’s being done to improve matters?

What is Healthcare Data Interoperability and Why Does It Matter for Clinical Research?
Read the white paper that examines how healthcare data interoperability is a critical requirement that impacts health outcomes, public health, and safety and has a major financial impact on everyone.

Solving the EHR to EDC Challenge – A Scalable-First Approach to
We all know about the data entry or re-entry burden our clinical trials place on our sites. Much of that data may already exist in the EHR (electronic health record) system. This white paper explores the importance of solving the EHR to EDC data re-entry challenge; why we’ve struggled to solve the problem at scale; how regulatory and technology tailwinds make a scalable solution possible; and introduces Medidata’s solution, Medidata Health Record Connect and Rave Companion.

Myth Busting: EHR to EDC
Moving data from Electronic Health Records (EHR) to Electronic Data Capture (EDC) systems in clinical trials involves several misconceptions that can complicate the process.
Watch how an EHR to EDC interoperability engine like Medidata Health Record Connect and Rave Companion work together ensuring clinical trial sites can easily and quickly populate Rave EDC forms with source data from other systems (e.g., EHR, CTMS, eSource) and documents (e.g., lab values in a spreadsheet).