Intelligent Trials
Design and Execute Faster Trials with AI-Powered Analytics
Performance Analytics, part of the Medidata Study Experience, helps clinical operations teams improve forecasting accuracy by tracking studies in real time and benchmarking against current industry performance. Powered by data from over 8,000 active studies, it supports early planning and live-study forecasting with country- and site-level insights, so you can detect enrollment risks earlier, modify plans quickly, and make confident decisions before timelines are at risk.
Benefits of Clinical Trial Analytics

Design Operationally Feasible Studies
Medidata Protocol Optimization, part of the Medidata Study Experience, transforms trial design and execution by leveraging AI-driven predictive modeling to simulate trial performance. This solution can predict the impact on patient burden, site performance, and costs well in advance of the First Patient In (FPI), resulting in research and operations teams gaining critical foresight into potential challenges. This approach significantly decreases costly amendments and enrollment delays, leading to smoother and lower-cost trials that are easier on patients and sites.
Identify Optimal Study Footprint
Assess historic enrollment rates from industry-wide trials to determine realistic timelines and study footprints. Then, analyze which countries and sites would be optimal for your study’s goals and timelines.
Select High-Performing Sites
Confidently select the right sites for your trial and target patient populations using AI-powered forecasting that uses actual site-level enrollment and congestion data, delivering greater accuracy and reliability for study planning.
Learn more about Intelligent Trials Study Feasibility.

Enroll More Diverse Patients
Find sites with a proven track record of recruiting diverse patients to ensure your trials reflect the diversity of real-world populations and meet regulatory diversity requirements. Our indication-specific, cross-industry patient demographics data allows you to identify and prioritize sites that consistently meet diversity goals, helping you achieve your inclusivity targets and drive equitable clinical outcomes.
Learn more about the Intelligent Trials Study Feasibility Diversity Module.
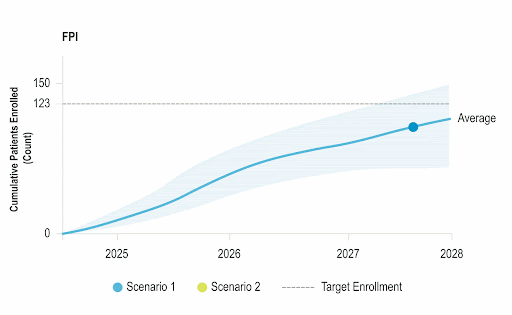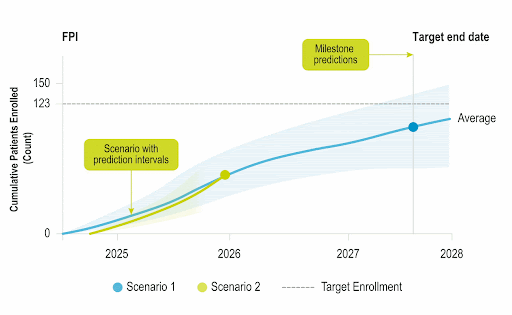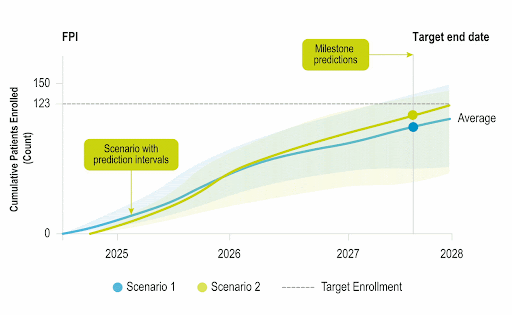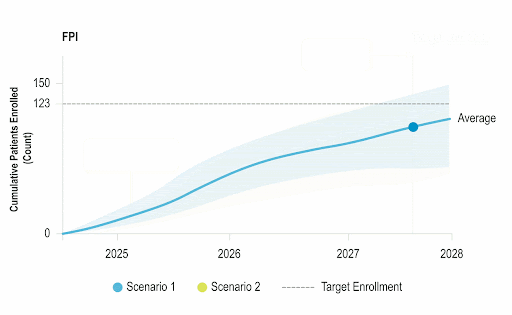
Stay Ahead of Enrollment Risks with Live Study Forecasting
Improve your forecasting accuracy by evaluating the progress of active studies using current performance data and industry benchmarks. Use near-real-time insights to monitor study health and detect enrollment risks early before they threaten your timelines
Key Features
One of the Industry’s Largest Clinical Trial Datasets
Gain insights from cross-industry clinical trial data spanning 36,000+ trials and 11 million patients across over 140+ countries to design and run more effective studies. Our AI-powered solutions transform site-, patient-, and indication-level data into clear, actionable insights, helping you model scenarios, mitigate risks, and make confident decisions at every stage of your trial.
Unmatched Site-Level Granularity
Utilize true site-level operational performance metrics – including enrollments, screen failures, data quality, competitive trials, and patient diversity– giving your team an unmatched, actionable view of each site’s current capabilities. Pinpoint high-performing sites that will help you hit your enrollment timelines, meet your diversity action plan goals, and give you a competitive advantage in a challenging recruitment landscape.

Actionable Real-Time Insights
Leverage data refreshed weekly from over eight thousand active studies to ensure your decisions are based on relevant, timely insights. This allows your teams to calibrate performance and take immediate action in a changing landscape.

Turnkey with White-Glove Support
Make confident decisions via access to off-the-shelf SaaS solutions, and available APIs combined with dedicated white-glove services support.
Learn More

Featured Whitepapers and Reports
The State of Black Participation in Clinical Trials
In our recent research report, we used Medidata’s industry-leading clinical trial data repository to assess the current state of racial diversity beyond the FDA Snapshot.

Featured Case Studies
Medidata Selected by Worldwide Clinical Trials to Accelerate Trials and Transform Patient Experience
Medidata and Worldwide Clinical Trials have expanded their partnership to leverage Medidata’s advanced solutions and extensive data set, enhancing Worldwide’s clinical operations with data-driven insights to accelerate trial timelines, improve site selection, and advance the life sciences industry.

Featured Podcasts and Webinars
ASCO Education: Racial Disparities in Clinical Trial Participation
Learn about the state of clinical trial diversity in clinical trials and what clinicians and trial sponsors can do to improve participant diversity.
Featured Blogs
3 Ways to Approach Challenges During Study Feasibility Processes
Industry data and predictive models, in real time, enable pharmaceutical companies and CROs to improve clinical trial site selection, forecast enrollment timelines, and optimize study feasibility through accurate scenario analysis and site performance predictions.