Adjudicate
Endpoint review often breaks down as handoffs increase and more reviewers get involved.
Medidata Adjudicate brings that work into one place, so your teams keep decisions consistent without slowing review timelines.
Ensure Every Endpoint Decision is Traceable
As endpoint volume increases, applying decisions consistently becomes harder—especially when more reviewers and committees are involved. Delays and rework often follow when review processes aren’t built to scale.
Adjudicate gives teams a structured way to manage endpoint review, helping decisions remain consistent and traceable, with the confidence needed for regulatory scrutiny.
Keep Endpoint Decisions Consistent
Track Event
Status in One Place
Minimize Review
Delays and Rework
Support Predictable
Review Timelines
Reduce Duplicate Entry
with EDC Integration
Where Adjudicate Makes a Difference
Structured Review
Standardize Endpoint Review
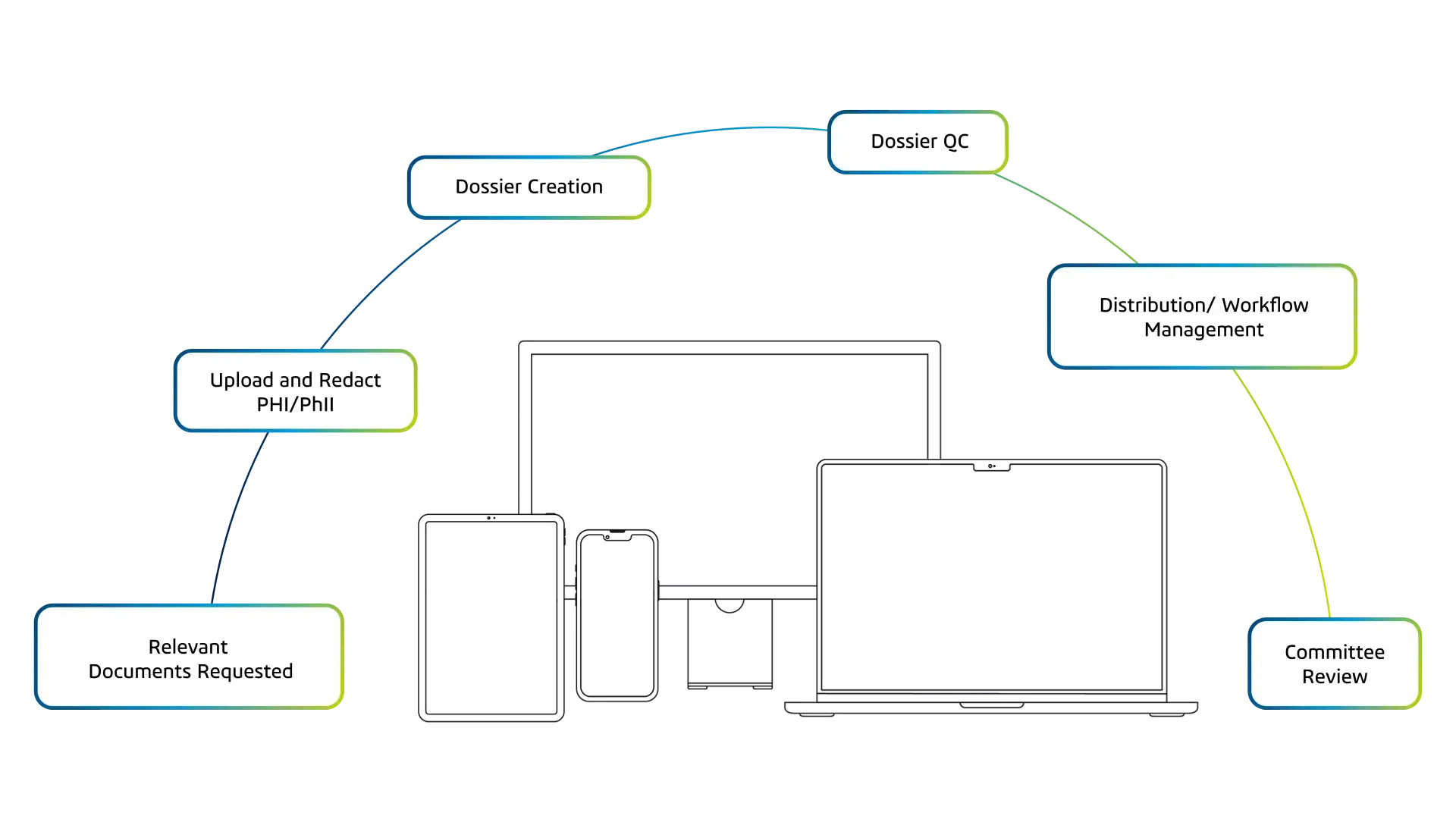
Adjudicate provides a structured way to manage endpoint review, from event intake and de-identification to dossier assembly and committee decision-making.
Teams apply endpoint definitions consistently, without relying on manual coordination or disconnected tools.

Featured Resource
Medidata Adjudicate Demo
See how Adjudicate supports structured endpoint review, event tracking, and committee decision-making in a single system.
Watch DemoFAQ
Explore Experiences
Discover the Medidata Platform