Medidata Clinical Trial Financial Management
Make Smarter Decisions with Data-Powered Financial Management
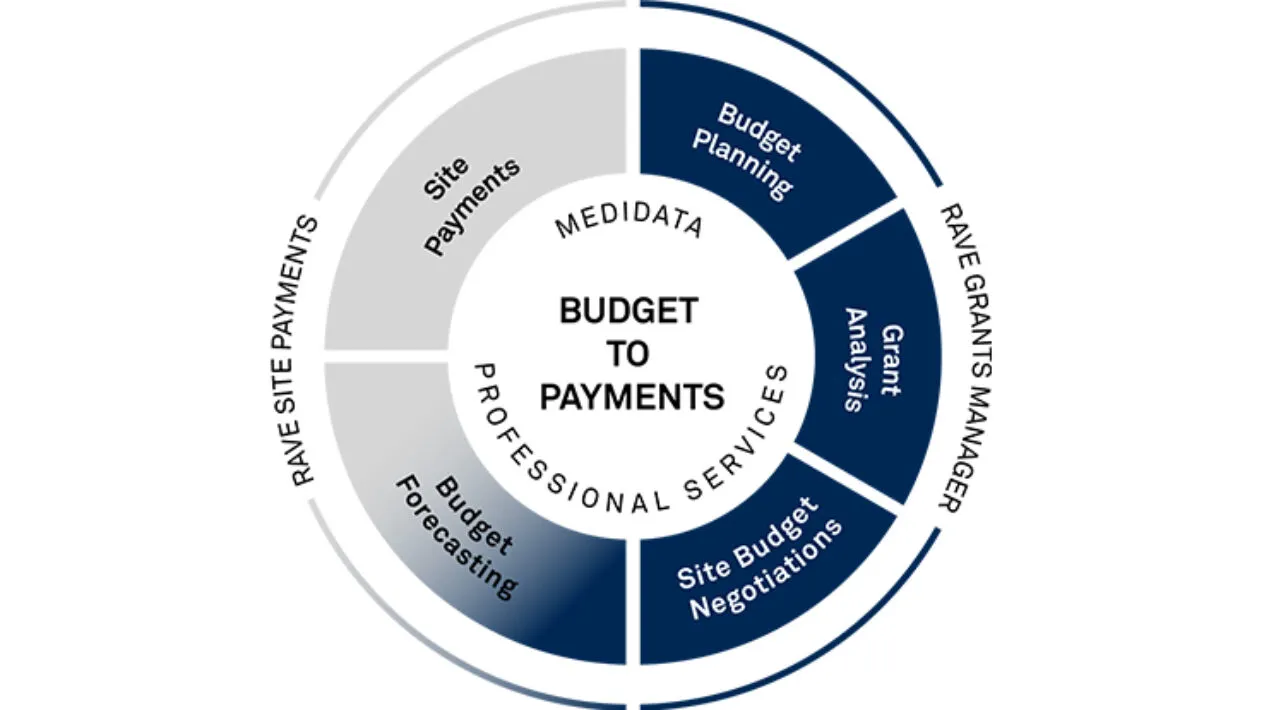
Medidata Clinical Trial Financial Management (CTFM), part of the Medidata Study Experience, is a suite of solutions that provides Sponsors and CROs comprehensive, best-in-class financial management solutions from study budget planning to site budget negotiations and payments.
Benefits
Improved Operational Efficiencies
Leverage automated workflows, dashboards and calculations that facilitate quicker site negotiation and site payment processes all to ensure study timeline targets are met and financial visibility is at the forefront.
Data Powered Decision Making
Take a faster and smarter approach to financial management with the unification of all study data and financial data. Utilize real time financial data tracking and analytics to make proactive and faster decisions.

Full Financial Transparency
Gain full transparency throughout the study lifecycle from study budget planning and site budget negotiation, to site payments and budget amendments, while providing sites the financial transparency they need.

Shift Focus Back to Patients
Enable sites to spend less time worrying about finances and more time conducting the study and focusing on the care of patients.
Key Features
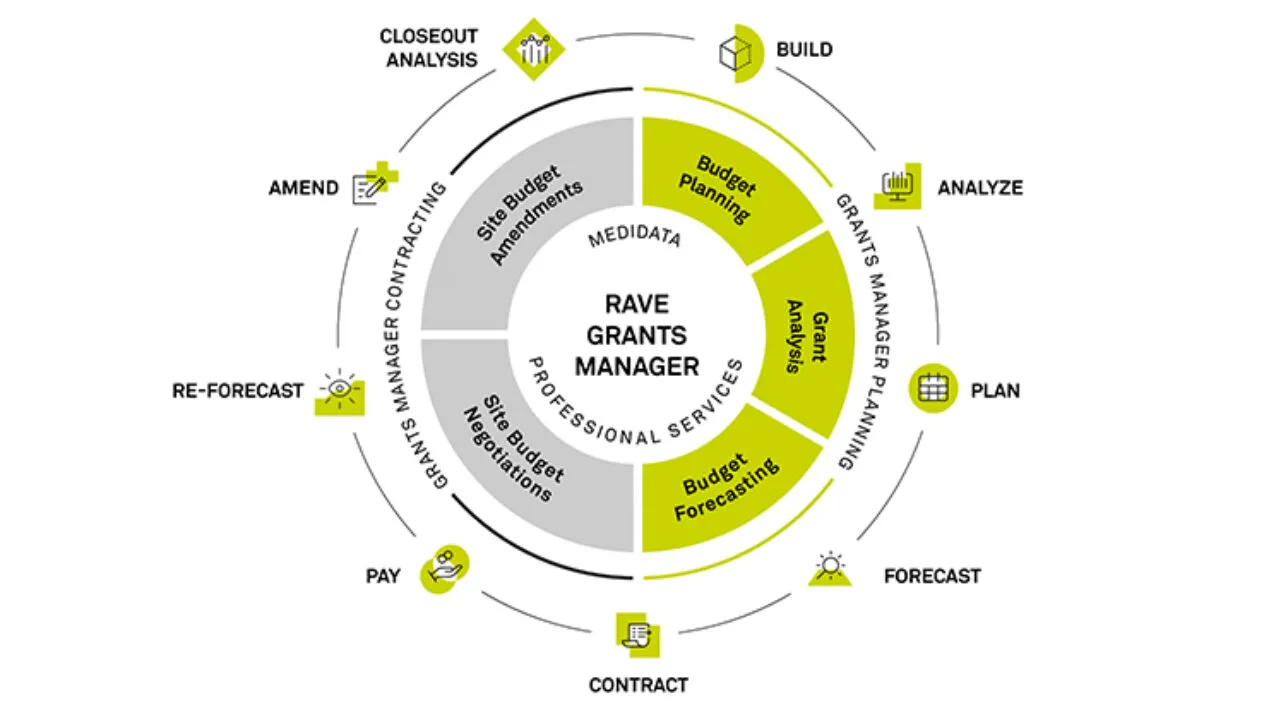
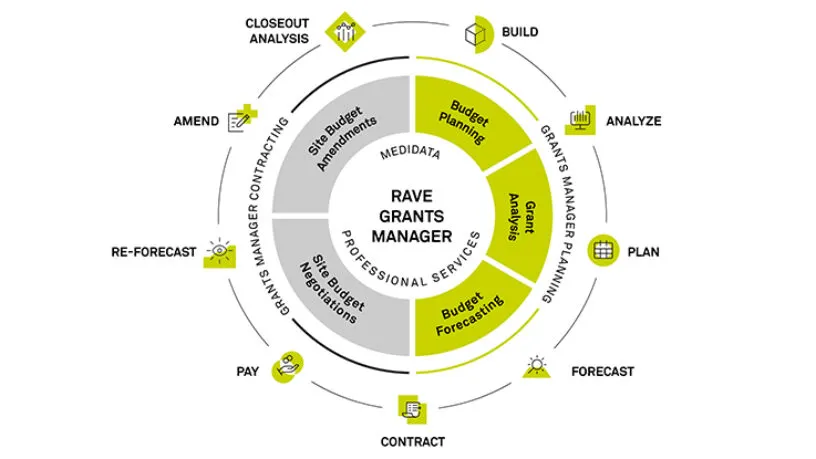
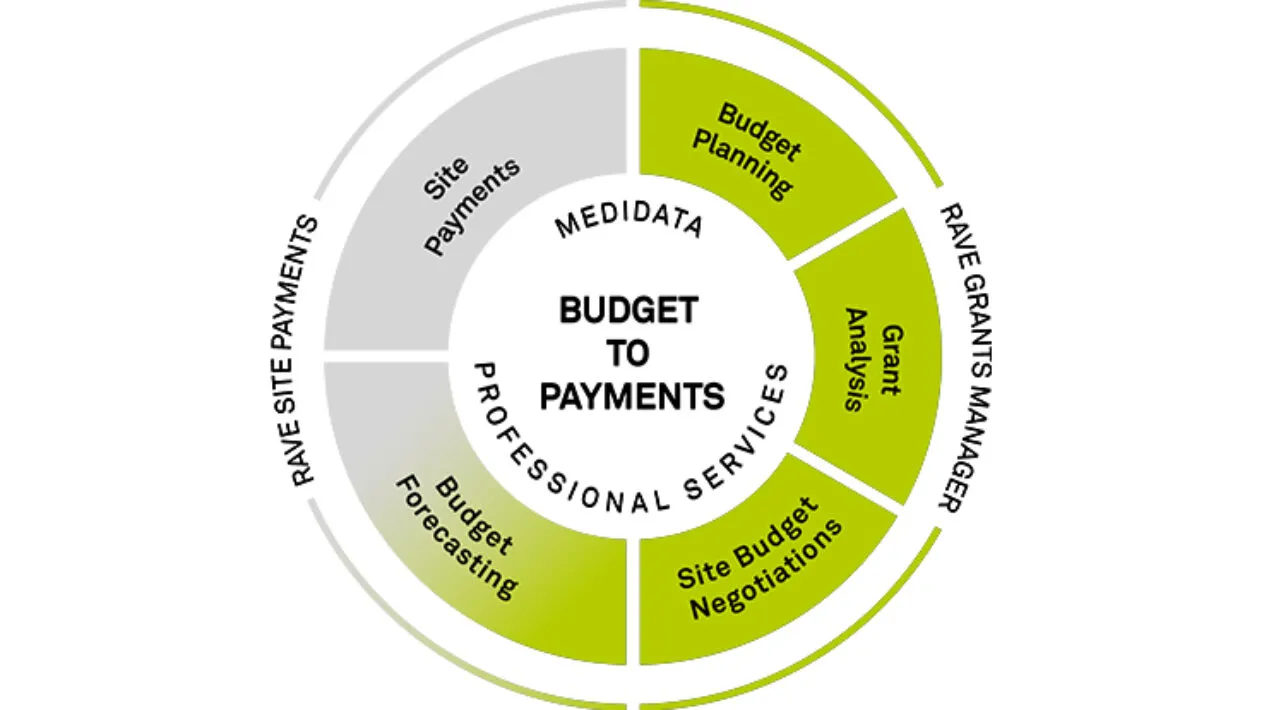
Study Budget Planning
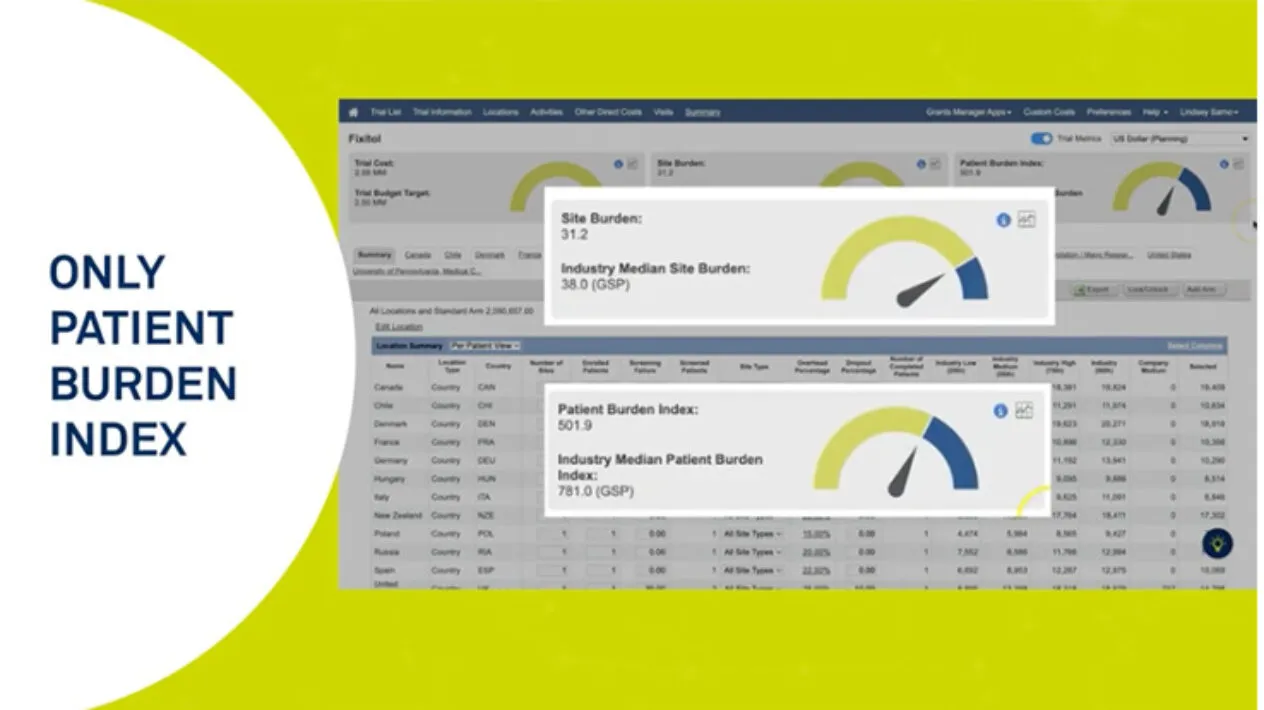
Rave Grants Manager Planning allows for a quick way to build a study budget with benchmarks running at every level of detail, from just the therapeutic area and phase, all the way to geographical and even site specific. The selection of different benchmarks allows for real-time scenario planning. It also provides a way to measure burden on sites and patients during the study budget build process.

Site Budget Negotiation
Rave Grants Manager Contracting automatically pulls the budget template from Rave Grants Manager Planning. It then powers site negotiations with automated, transparent workflows, interactive dashboards, metrics and tracking to enable quick and easy budget negotiations with the sites.
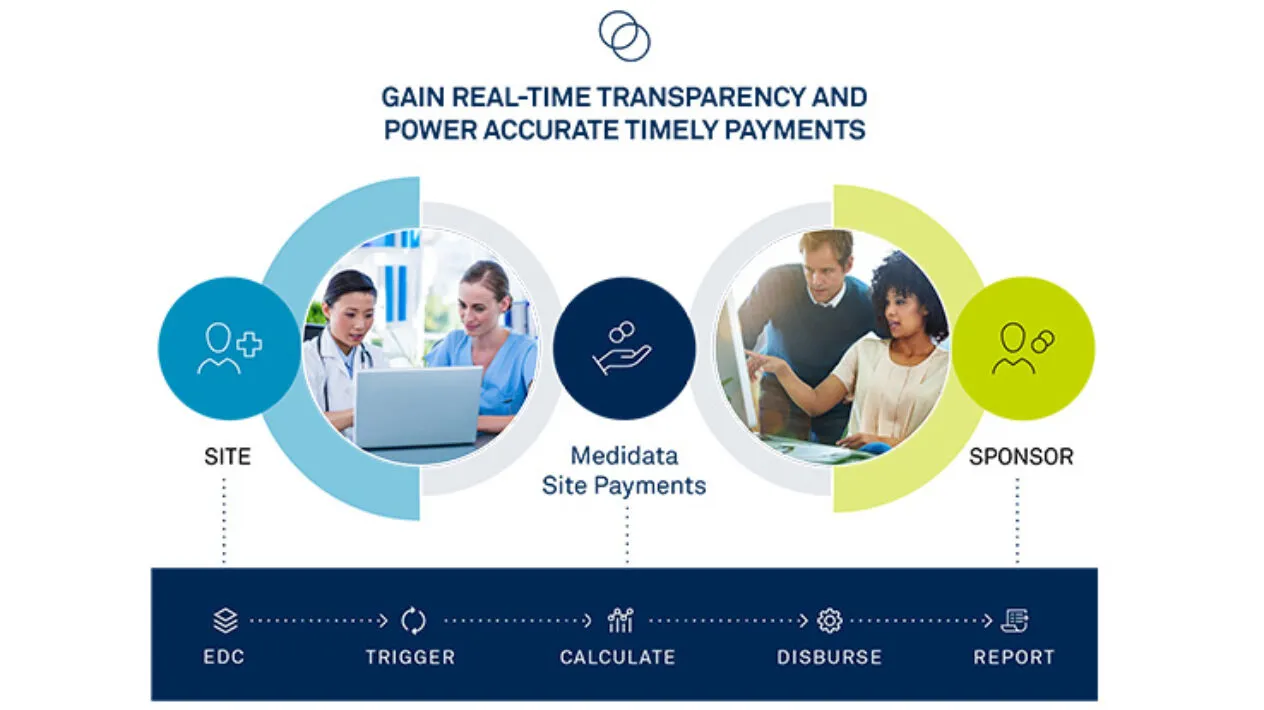
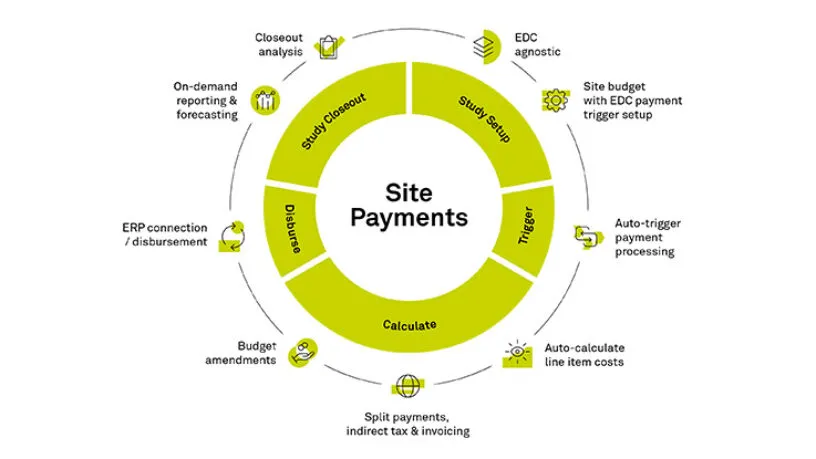
Accurate & On-Time Site Payments
Rave Site Payments handles complex budgets and payment terms with automated cost calculations triggered by Rave EDC in real-time or any other EDC solution. Streamlined and controlled payment approval queues and funding processes eliminates payment delays.

Site Portal
The site portal provides complete visibility into the budget negotiation and payment processes. Site portal dashboards, reports & auto invoicing allow sites to conduct adequate financial planning, resulting in increased financial stability.

Data-Powered Reporting & Analytics
Unified data powers smart accrual tracking and forecasting that enables proactive financial decision making while obtaining complete visibility and meeting all compliance requirements.
Robust Global Invoicing
Rave Site Payments reduces processing time and errors with the global invoicing module that automates and distributes electronic invoices while taking the burden off the sites.
Related Solutions
Resources

Clinical Trial Financial Management
From study budget planning, to site budget negotiations and site payments, Rave Clinical Trial Financial Management ensures financial visibility for all stakeholders throughout the entire study life cycle. Read about all the features included within the suite of products.

White Paper: Better Data, Better Decisions
Discover how Medidata addresses the need for accurate and defensible trial budget costs. This article explores a multidimensional method to determine market-based costs.

POP Magazine
Check out the Power of Partnership (POP) quarterly magazine. Read the latest issues with timely and compelling clinical finance news and information. Sign up to receive the magazine.