Grant Management Software for Clinical Trials
Smart Clinical Trial Budgeting with Better Data
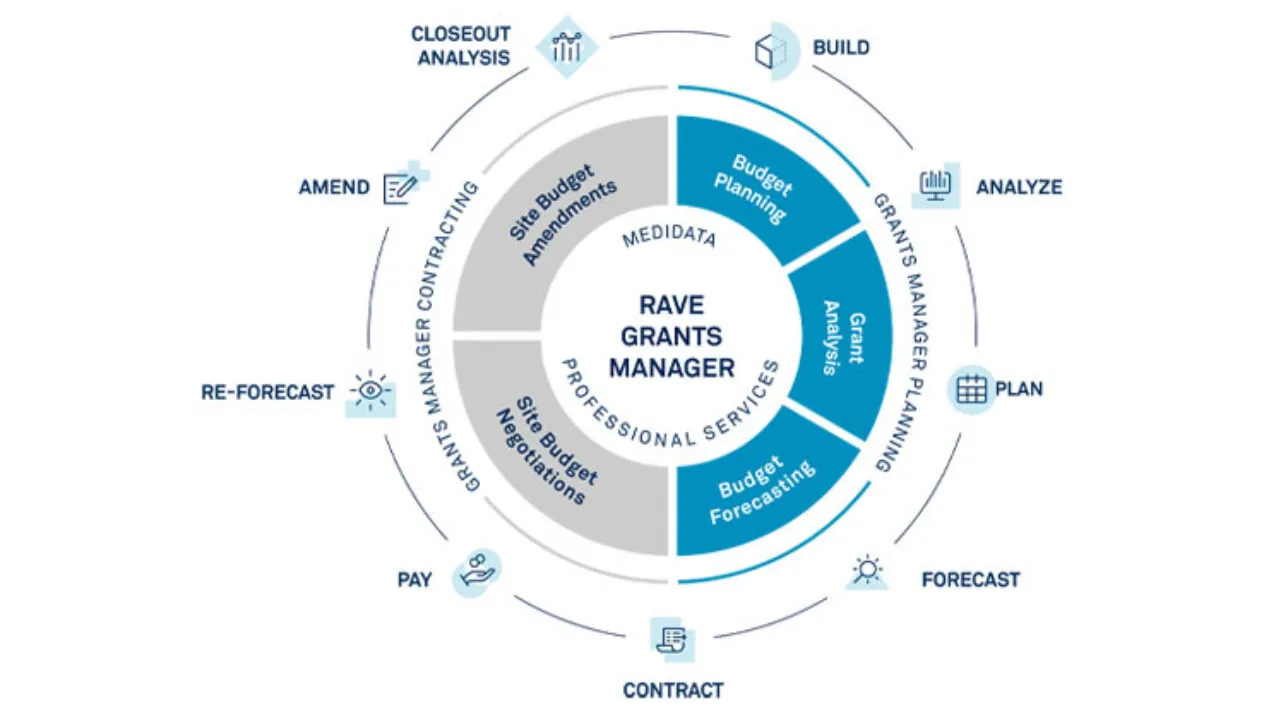
Rave Grants Manager, part of the Medidata Study Experience, manages the entire investigator grant life cycle of clinical trials. The solution provides Sponsors and CROs with a comprehensive, data-driven way to quickly and accurately develop investigator grant budgets and efficiently conduct the site budget negotiation process.
Key Benefits

Optimal Planning & Execution
Make better decisions to meet your financial goals.

Financial Scenario Planning
Access predictive costs to understand future Fair Market Value(FMV).

Better Site Relationships
Ensure fair site budgets that establish trust.

Effective Financial Analysis
Identify key drivers across committed, anticipated, and projected costs.
Key Features

Streamline site budget negotiations with automated budget template generation, workflows, audit tracking and comprehensive dashboards.
Quickly run a study budget forecast and access your study-specific historical budget data within one repository.
Quickly look up a cost benchmark on demand.
Study Complexity and Site & Patient Burden scores are automatically calculated within the Grants Manager dashboard as you build your budget.
Dynamic Fair Market Value (FMV)
Refine the accuracy of your investigator grant budget by leveraging insights with the right data. By harnessing AI, Rave Grants Manager adeptly forecasts FMV, accounting for dynamic variables, ensuring both transparency and protection of your site budgets from future uncertainties. Its predictive capabilities go beyond traditional analysis, delivering a detailed, data-driven perspective on anticipated financial trends.
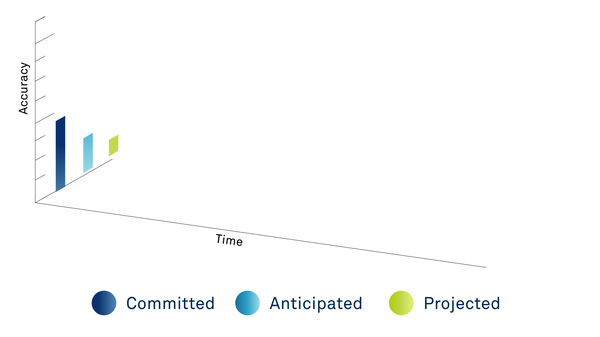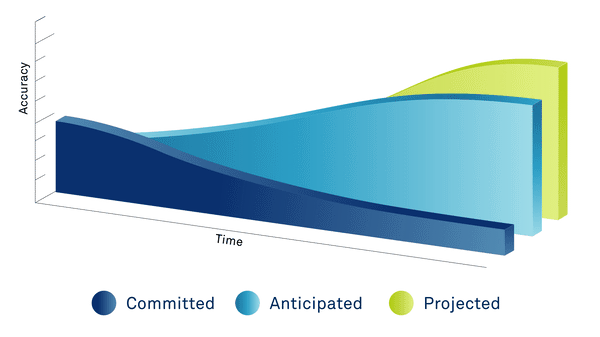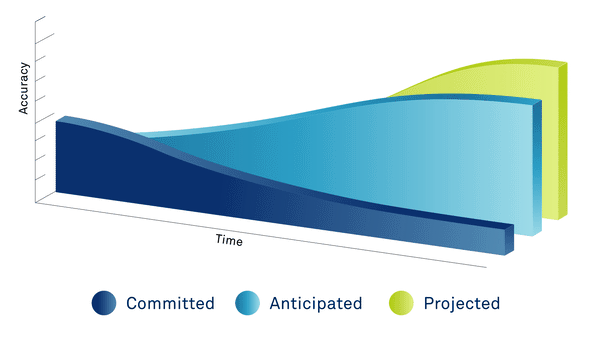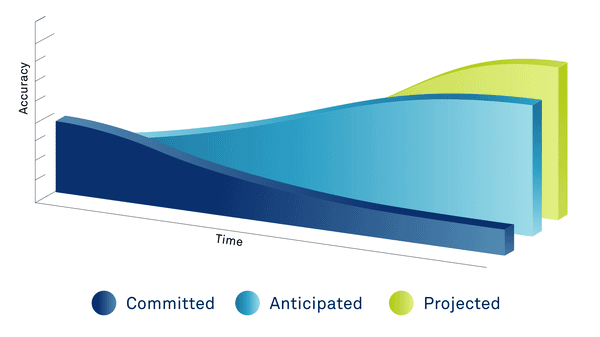
PAST
Data extracted from executed site contracts

PRESENT
Committed data + 3rd party data sources, country ratios, exchange rates, inflation & statistical models

FUTURE
Predicted data based on the length of the study
Financial Scenario Planning
Be Data-Informed and Take Control of Your Clinical Trial Budgets
Medidata Financial Scenario Planning is your complete solution for clinical trial budget planning. This advanced solution helps navigate the complex financial landscape with confidence and precision. Leverage advanced analytics and real-time data for scenario modeling, forecasting outcomes, and informed decision-making that all ensure sustainable growth and complete budget control. Financial Scenario Planning significantly reduces uncertainties in financial, economic, and market conditions specific to clinical trials. Redefine what an “accurate budget” means for your future trials.
Agile Scenario Modeling
Enhance business agility with dynamic planning that integrates strategy, operations, and finance for rapid adaptation to market shifts.

Risk Mitigation
Proactively identify and simulate financial risks using insights for informed and effective future planning.

Seamless Regulatory Compliance
Streamline compliance with automated workflows designed to reduce financial risk.
Learn More

Better Data, Better Decisions
Achieve greater certainty in clinical trial financial management by leveraging adaptive, accurate, and defendable fair market value.

Transform your Site Budgeting Process
Revolutionize budget planning and contracting with Rave Grants Manager Contracting. Automate negotiations, gain full visibility and boost efficiency for Sponsors, CROs, and sites.

Data Powered Financial Management for Your Clinical Trials
Achieve complete financial visibility, eliminate complexity, and ensure the financial health of your clinical trials with Rave Clinical Trial Financial Management.



