ICYMI Vol 1 Issue 2

Welcome to the 2nd edition of Medidata’s partner bi-quarterly newsletter, In Case You Missed It (ICYMI). In this issue, we cover the following topics:
- How to Update Your Company Profile
- Patient Cloud Solution Proposals
- Product Updates – Site Cloud EOS, Intelligent Trials Diversity Module
- Changes to Medidata Detect Quote Requests
- Webinar Series on Data & Analytics Reporting
PRODUCT UPDATES
The 90's Called ... It Wants Its CDs Back!

Site Cloud: End of Study (EOS) is the first end-to-end solution that seamlessly generates, distributes & manages sites’ study files at the end of a study. With EOS, sites’ study files are accessible and downloadable via a secure and trusted unified platform, eliminating the need to create and distribute physical media and deal with paper acknowledgment forms. EOS is transforming the way sites’ study files are collected and stored.
Here's the fact sheet about Site Cloud: End of Study (EOS) as well as a case study on how a biotech reduced regulatory compliance risks with the ability to track 100% of sites receiving data. This is compared to the previous workflow where approximately 30% to 40% of sites would neglect to notify the company that they had received the media. The company now receives updates from all sites through automated audit trails that indicate when sites receive data.
Diversity Module Now Available in Intelligent Trial Study Feasibility Tool
Medidata has launched the Intelligent Trials Diversity Module, a new tool to help improve equity in clinical trials by providing site-level patient demographic data including race, sex, age, and ethnicity. The diversity tool can baseline and benchmark the diversity of trials in a specific indication vs. industry trials to identify gaps and reasonable diversity goals, providing actionable insights into how to improve the diversity of trials by identifying sites that are more likely to perform well operationally AND recruit diverse patient populations.
View this page to learn more about Intelligent Trials and the benefits of clinical trial analytics, For the Fact Sheet/Use Case, click here
COMMERCIAL DESK
Medidata Patient Cloud Solution Proposals
Did you know Medidata can provide you with proposal-ready content for any and all Patient Cloud Solutions, such as eCOA App, eConsent App, Sensor Cloud, as well as the entire suite of myMedidata web-based DCT offerings (myMedidata eCOA, myMedidata eConsent, myMedidata LIVE (Telehealth), myMedidata Registries)? This content enables you to quickly complete, configure, and customize each of your client proposals. Proposal content includes solution overviews, experience metrics, implementation process details, shipping and regulatory risks/mitigations, timelines, etc.
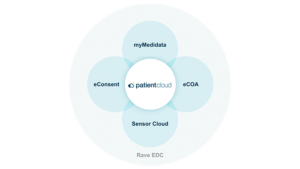
We encourage you to let us help you build your own collection of Medidata proposal-ready content, demonstrating the value you bring to your clients in our partnership. Contact your Medidata Partner Account Manager (PAM) or Partner Business Manager (PBM) for more details.
TRAINING & CERTIFICATION
Global Education
To support experienced learners who need to learn only about new features introduced in a specific release, Release Training eLearnings are available on iMedidata. Over the past year, we’ve made Release Trainings more accessible by also linking them to the product Knowledge Spaces.
These NEW eLearning courses are now available via iMedidata:
- Site Cloud: End of Study (EOS)
- Coder + Task Management/ Dictionary Management/Browse & Code
- Rave CTMS Configuration Management
Updates Available:
- eLearnings – TSDV and Remote Source Review (RSR)
- Show Me Video – Site Cloud: End of Study (EOS)
Click here for the course catalog. If you have questions about these offerings, please contact the team at medidata.globaleducation@3ds.com
PARTNER PROGRAM

Webinar Series on the Power of Medidata's Data & Analytics
Are your audit reports failing or timing out? Is consolidating data from disparate systems inefficient in your workflow? Join these sessions and get insights on how to leverage the Medidata platform to save time and become more efficient with your data.
Access to Data in the Data Fabric | Nov 3 @ 10:30am ET | REGISTER
Gary Luck, Sr. Director of Data Platform, will focus on the methods for helping you and your clients access data in current and future states.
Leveraging Current and Future Reporting Analytics Capabilities | Nov 10 @ 10:30am ET | REGISTER
Dave John, Sr. Director of Data Visualization, will show you the current reporting solutions and how to leverage them for your reporting needs today. He will also provide a demonstration into the future of analytics and reporting.
PartnerConnect Corner: Medidata Detect Quote Request Changes
We have made some changes to Medidata Detect. Medidata Detect is a comprehensive data and risk surveillance solution which now includes modular, fit-for-purpose capabilities. Detect’s capabilities cater to multiple end users including data managers, medical monitors, central monitors, CRAs, and project/program managers. Users can now select the analyses and features for their specific study needs. The four modules within Detect are Patient Data Surveillance, KRIs, QTLs, and Centralized Statistical Monitoring. Learn more about these changes here
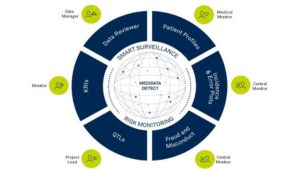
As a result of these changes, we have also updated our Quote Request form with the following:
- Removed Patient Profiles question - Medidata Detect Patient Data Surveillance module now includes Patient Profiles and Data Reviewer
- Addition of a new field allowing the Partner user to select the relevant Medidata Detect PDS and/or KRI modules (new SKUs) along with the relevant Help Text/Hint - Medidata Detect now includes fit-for-purpose modules developed to fit specific user activities and analyses
- Update of the Help Text/Hint for the Checkbox to select Medidata Detect giving you information on what is needed for this field
Quick Tips
Have you updated your company profile page recently? If you haven’t reviewed it in a while, it might be a good time to ensure the profile is representing your company today – perhaps include your experience with Medidata solutions, and include recent accreditations. Please submit any updates via this form. If you have questions, reach out to medidata.partners@3ds.com.
AWARDS & RECOGNITION
Partner Accreditations
Congratulations to Biotrial and eClinical for achieving new accreditations!


Partner Anniversaries
Thank you eClinical for 15 years of partnership, and to Medpace and Rho for 10 years of partnership!
NEWS & EVENTS
WHAT TO LOOK FOR IN THE NEXT EDITION
- Additional services available for your eCOA proposals
- Learn how PartnerConnect can help you Win More with the Medidata Resource Hub!
- Rave EDC Adoption Update
- Learn more about Rave Companion
- OnDemand Recordings for Data & Analytics Webinar Series
Finally, we welcome comments and suggestions for future topics. Our goal is Connecting with Partners to Solve the Impossible. We will continue to find new ways to grow and cultivate a leading partner program for you! Please contact us via email medidata.partners@3ds.com
