AI in Clinical Study Builds: Redefining EDC Efficiency

Complexity is synonymous with clinical research. But the word ‘complex’ has never been more appropriate than it is now as the industry leaps forward with transformative advancements in research methodologies, centralized frameworks, technologies, and data interoperability.
As clinical data continues to grow in complexity, volume, and velocity, the demands on clinical trial technologies have increased, including EDC systems that act as the central source for that data. Advances in AI and technology have greatly assisted in automating manual processes and limiting risks—with new developments taking that further.
Medidata has been leading advancements in clinical trial technology for decades, and 2025 has seen groundbreaking releases that bring researchers closer to a 360° AI–powered clinical trial environment.
Medidata Rave EDC is the heart of the Medidata Platform, which is underpinned by AI and the industry’s most advanced data management architecture.
Marco Amorim, Senior Director, Product Management, Medidata, shared insights into how Medidata Designer uses AI to transform study builds and reduce timelines from 10-12 weeks to just a few days.
The main reasons for lengthy lead times are:
- Protocol Complexity: Translating clinical protocols into operational elements involves interpreting complex requirements, defining every data point, designing intuitive forms, implementing complex validation rules, and configuring the database structure.
- Manual Processes: Manual CRF and edit check creation can be labor-intensive and resource-constrained.
- Collaboration Bottlenecks: Cross-functional review cycles (involving data managers, clinical teams, programmers, etc.), testing, and adjustments lead to amendments and delays and introduce the risk of compromised data integrity.
- Validation & Testing: CRFs, edit checks, and workflows must undergo multiple rounds of validation & testing.
Laying the Foundation: Study Design Essentials Using AI in EDC Systems
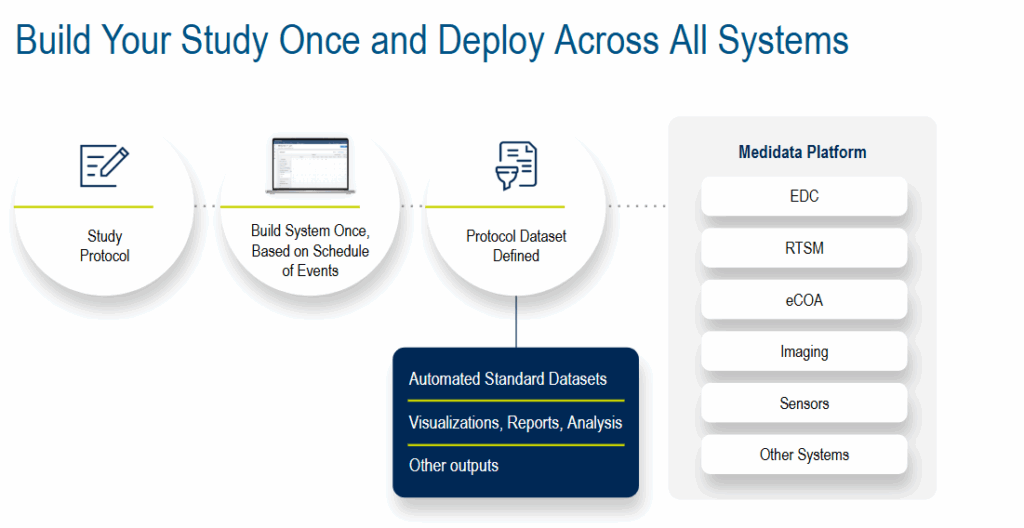
A strong foundation is critical to clinical trial success. Medidata Designer takes a “build once, deploy across all systems” strategy. An AI framework within the EDC study build process achieves this and more, turning static protocol documents into dynamic data collection structures.
Protocol requirements, such as inclusion criteria, visit schedules, CRFs, etc., are translated into the EDC build. Then, AI predictive design engines improve accuracy by suggesting form structures based on study type, therapeutic area, and/or historical builds. Smart standardization tools automatically map data fields to CDASH or SDTM variables, reducing manual reconciliation.
This transforms labor-intensive, reactive tasks into faster, proactive, and intelligent automated processes.
Examples include:
- Intelligent Protocol to eCRF or Statistical Analysis Plan (SAP) Translation: Natural language processing (NLP) analyzes study protocols, identifying key data elements, visit schedules, and study objectives. AI automatically generates initial eCRF or SAP drafts and suggests optimal form layouts and data collection flows based on historical trial data and best practices.
- Predictive Validation and Smart Edit Checks: Manual coding of each validation rule is replaced by AI algorithms that can learn from the vast datasets of previous trials to predict common data inconsistencies and suggest relevant edit checks. This accelerates the study set-up and enhances data quality by identifying potential errors. Unusual data patterns are flagged in real-time during data entry, prompting immediate review and correction.
- Optimized Database Configuration: AI analyzes the protocol and eCRF design to automatically configure the database structure. This ensures optimal data storage, retrieval, and integration with other systems and minimizes extensive manual database programming and integration challenges.
- Enhanced User Acceptance Testing (UAT): AI can assist human oversight in UATs by simulating data-entry scenarios and identifying potential usability issues or gaps in validation rules. This enables efficient EDC build refinements before the study go-live.
- Adaptive Trial Design Support: As a trial progresses, AI analyzes accumulating data, suggesting real-time adjustments to the protocol or eCRFs, supporting adaptive trial design. This flexibility, based on emerging insights, can accelerate drug development by optimizing dosage, patient populations, or study endpoints.
By automating repetitive tasks, AI enhances data quality at the source, provides predictive insights, and empowers researchers to focus on higher-value activities.
Case Report Forms, Rules, and Logic: Building Smart Forms with Smarter AI
CRFs are at the heart of data capture in clinical trials, crucial for compliance and ensuring high-quality data.
Traditional form building involves selecting fields, configuring logic, and adding edit checks. As study complexity increases, so does the risk of errors, redundancies, and inconsistencies. Best practices include modular form design, reuse of standardized components, and rule libraries to streamline validations.
AI empowers study builders with faster build cycles, fewer protocol deviations, and better user experiences for sites and monitors through:
- Form auto-generation from protocol text, historical templates, and therapeutic area-specific standards.
- AI-assisted logic design that suggests edit checks, skips patterns, and calculates fields based on prior builds or therapeutic knowledge.
- Machine learning models that identify patterns of frequent data entry errors and recommend improvements to form design before deployment.
From Draft to Database: Testing, UATs, and Go-Live in the AI Era
Once the forms are built and the logic is in place, the next step is to validate and deploy the study. This involves internal testing, UATs, and go-live preparations.
Testing ensures the study functions as intended–that edit checks fire correctly, CRFs load as expected, and all logic works under real-world conditions. The UAT confirms that site users and sponsors are comfortable with the interface and workflow.
AI tools minimize the burden on QA teams through:
- Auto-generated synthetic test data creation, which reduces the time taken to generate CRF-specific test data for integration, edit checks, and UAT from days to minutes. It can also provide a scenario simulation before study go-live.
- Anomaly detection algorithms that flag inconsistent or illogical configurations pre-UAT.
- AI-assisted UAT scripting and validation documentation.
- Interactive guides or chatbots to support user onboarding.
- Post-go-live monitoring that predicts protocol amendments or mid-study changes and evaluates the impact before implementation.
Conclusion
The examples in this blog put this into context, and a deeper understanding of complexity in clinical trials can be gained through discussions with Medidata or by accessing any of our many resources.
The Association for Clinical Data Management (ACDM) and Society for Clinical Data Management (SCDM) websites also provide insights into the levels of complexity involved in each step of a study’s lifecycle—from strategy and planning to study set up, conduct, and closeout stages.
Traditional technologies, such as standard EDC systems, siloed working practices, and disjointed frameworks and methodologies struggle to be fit for purpose as we usher in a new era of clinical research.
The need for an AI-powered 360° clinical environment within a centralized framework and methodology is clear. Building on the fundamentals of good study design, AI is redefining everything from protocol parsing to CRF creation and system validation.
Intelligent automation is now an essential tool for modern clinical trial operations, aligning with the goal of delivering safe, effective therapies to patients sooner.
Medidata Designer is part of that next phase in the clinical research AI revolution, making study building within EDC simpler and faster while improving study build quality and promoting/increasing the use of standards.
Contact Us

