CNS Clinical Trials 101: Everything You Need to Know

“Alzheimer’s is the cleverest thief, because she not only steals from you, but she steals the very thing you need to remember what’s been stolen.”
– Jarod Kintz1
For patients living with neurological or neurodegenerative disorders, memory loss is a devastating erosion of identity. Diseases that affect the central nervous system (CNS), like Alzheimer’s, chip away at a person’s sense of self—long before their physical body shows signs of decline.
This existential experience is what drives the urgency behind CNS clinical trials. More than 3 billion people worldwide are affected by neurological disease2, underscoring the scale of the challenge. In Alzheimer’s research alone, a Phase III trial averages $370 million to run3, may take up to eight years to complete for preclinical Alzheimer’s Disease from study start up to protocol completion4, and risks a 95% failure rate among drug candidates aimed at slowing or modifying disease progression5.
A host of factors feed into these significant challenges, including complex and subjective endpoints, high screen failure rates, and high placebo response rates.

These numbers show why CNS clinical research has some of the most complex studies compared to other therapeutic areas. It requires specialized assessments, rigorous consistency in data capture, and a patient and site-centric approach that accounts for cognitive, behavioral, and emotional challenges.
Read on to explore the foundations of CNS clinical trials, common challenges, and how innovations like electronic clinical outcome assessments (eCOA) address long-standing pain points in data quality and trial execution.
Why are CNS Trials Complex?
CNS trials involve evaluating new treatments, devices, or interventions that affect the brain and spinal cord. These trials play a vital role in advancing care for conditions like Alzheimer’s disease, schizophrenia, depression, and other CNS disorders.
Because CNS disorders often affect cognition, memory, and behavior, both patient participation and study execution can be difficult. Patients can suffer from significant cognitive and physical impairments and may struggle with lengthy and challenging assessments, traveling to sites, or adhering to complex visit schedules. Clinical sites, on the other hand, must deliver accurate and consistent evaluations despite challenges like rater shortages, time shortages, and the looming risk of audit findings from misadministration or scoring errors.
Raters are trained clinical professionals, including psychologists, neurologists, psychometrists, nurses, or professionals with master’s-level clinical training, who are responsible for administering and scoring clinical outcome assessments (COAs) in CNS trials. Their role is critical, as they help make sure complex symptoms are evaluated with accuracy and consistency across all study participants.
The Role of eCOA in CNS Clinical Trials
Clinical outcome assessments (COAs) are instruments used to measure and capture how a patient feels and functions6. In CNS trials, these tools are essential for evaluating cognitive and functional change over time. There are four major types of COA, which include patient reported outcomes (PRO), clinician reported outcomes (ClinRO), observer reported outcomes (ObsRO), and performance outcomes (PerfO)6. When administered electronically through devices such as provisioned devices, tablets, web-based solutions, or patients’ mobile devices, they’re referred to as electronic clinical outcome assessments (eCOA).
Many CNS clinical studies rely on specific ClinROs that are used to assess cognitive performance and disease progression. For example, some of the most widely used assessments for Alzheimer’s Disease clinical trials include:
- Alzheimer’s Disease Assessment Scale - Cognitive Subscale (ADAS-Cog):
A gold-standard assessment tool used to evaluate cognitive symptoms in Alzheimer’s disease, including memory, language, and praxis7. - Montreal Cognitive Assessment (MoCA):
A brief screening tool for detecting mild cognitive impairment (MCI), assessing domains such as attention, memory, language, and executive function8. - Mini-Mental State Examination (MMSE):
A commonly used 30-point questionnaire to assess general cognitive impairment, assessing orientation, immediate and short-term memory, attention, language, and visuospatial skills9. - Clinical Dementia Rating (CDR):
A structured interview and assessment used to quantify the severity of dementia, combining clinician observation and caregiver input10.

Ensuring consistency and accuracy in these assessments is critical, which is why studies often rely on rigorous rater training and prompt feedback on scale administration and scoring—challenges that eCOA clinical trials are built to address. Modern eCOA technology for CNS trials has also evolved through close collaborations with clinicians and scientific experts. As a result, features like flexible navigation, advanced annotation tools, audio recording, automated transcription, and embedded clinical guidance have been thoughtfully incorporated—not only to reduce the rater burden when administering ClinROs, but also to drive higher data quality across CNS studies.
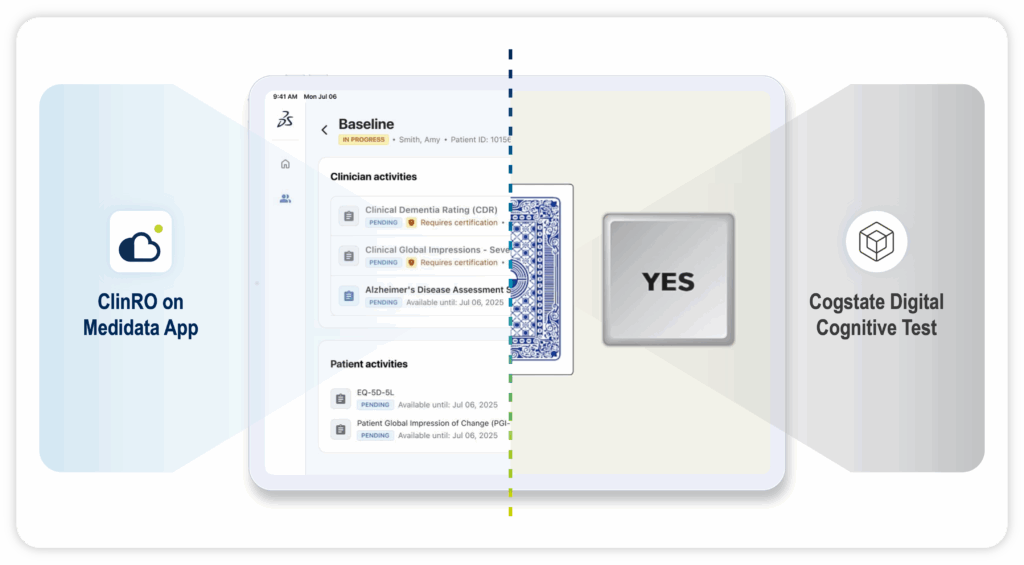
Complementing ClinROs with Digital Cognitive Assessments
ClinROs offer critical expert evaluation of cognitive function—particularly when clinical judgment is required to interpret complex patient behaviors and disease progression. Digital cognitive assessments, on the other hand, provide objective, performance-based measures that can sensitively detect changes in specific cognitive domains.
Depending on the trial’s design, disease stage, and regulatory goals, ClinROs and digital cognitive assessments can be used individually or in combination to optimize endpoint robustness, enhance sensitivity to treatment effects, reduce measurement variability, and give a deeper insight into a patient’s experience of their disease and treatments.
Digital cognitive assessments involve tasks that patients complete to measure specific cognitive functions like attention, memory, or executive processing11. These assessments, when digitized, can capture subtle changes that may not be easily observed or reported. Today, digital cognitive assessments are being used in CNS trials to:
- Detect cognitive decline earlier and more sensitively, with demonstrated responsiveness to drug-related change
- Enable standardized administration and real-time scoring without requiring expert neuropsychologist raters
- Eliminate variance due to rater discrepancies and remove the need for extensive rater training
- Support decentralized trial models with brief, repeatable tasks that are well-accepted by participants
- Maintain validity across languages and cultures
- Drive operational efficiency and consistency across sites
By integrating both ClinROs and digital cognitive assessments within a CNS trial, researchers can better track disease progression and improve endpoint robustness—all while reducing operational burden and enhancing the patient and site experience.
Looking Ahead: The Future of CNS Trials
One of the most exciting prospects in CNS trials lies in the integration of artificial intelligence (AI) into the already-established data quality assurance processes. By analyzing subtle speech patterns, such as repeated pauses, prolonged “ums” and “uhs,” and reduced pitch variability, AI can provide real-time insights into a patient’s cognitive state and disease progression. In parallel, machine learning algorithms can detect discrepancies in scale administration and scoring, minimizing rater errors and enhancing data integrity. These technological advances would act in tandem with the work completed by raters and reviewers to bolster the reliability of data captured and would act as another component in teams’ endpoint data quality programs.
CNS clinical trials sit at the intersection of medical science and human experience. Behind every data point is a patient navigating the emotional and cognitive challenges of their condition, and a dedicated site team working to capture that experience with care and precision—while medically and emotionally supporting the patient as best they can. That’s why the future of CNS research depends not only on scientific rigor but also on tools and technologies that honor the complexity of the diseases and the people affected by them. From eCOA and digital cognitive assessments to emerging AI-powered CNS clinical trial solutions, researchers now have more options than ever to elevate endpoint data quality, reduce operational burden, and make trials more accessible.
Through our award-winning partnership, Medidata and Cogstate are helping lead a new chapter in CNS research. By combining scientific expertise, purpose-built CNS solutions, and a deep focus on the patient and site experience, we’re transforming how patients’ cognitive function is captured, analyzed, and acted upon.

Discover how to optimize CNS assessments and endpoint data quality.
References:
- Kintz, J. (2018, November 4). Alzheimer’s: A different kind of thief. The Next Phase Blog. https://aknextphase.com/alzheimers-different-kind-thief
- Institute for Health Metrics and Evaluation. The Lancet Neurology: Neurological conditions now leading cause of ill health and disability globally, affecting 3.4 billion people worldwide. Published March 14, 2024. Accessed March 13, 2025. https://www.healthdata.org/news-events/newsroom/news-releases/lancet-neurology-neurological-conditions-now-leading-cause-ill
- Boxer AL, Sperling R. Accelerating Alzheimer’s therapeutic development: The past and future of clinical trials. Cell. 2023;186(22):4757-4772. doi:10.1016/j.cell.2023.09.023
- Goldman, D., Malzbender, K., Lavin-Mena, L., Hughes, L., Bose, N., & Patel, D. (2020, August 17). Key barriers to clinical trials for Alzheimer’s disease. USC Schaeffer Center for Health Policy & Economics. https://schaeffer.usc.edu/research/key-barriers-for-clinical-trials-for-alzheimers-disease/
- Cummings JL, Lee G, Ritter A, et al. Alzheimer’s disease drug development pipeline: 2023. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2023;9(1):1-10. doi:10.1002/trc2.12388.
- FDA. Clinical Outcome Assessment (COA): Frequently Asked Questions. FDA.gov. December 2020. Accessed July 19, 2024. https://www.fda.gov/about-fda/clinical-outcome-assessment-coa-frequently-asked-questions#COADefinition
- Rosen, W. G., Mohs, R. C., & Davis, K. L. (1984). A new rating scale for Alzheimer's disease. American Journal of Psychiatry, 141(11), 1356–1364. https://doi.org/10.1176/ajp.141.11.1356
- Nasreddine, Z. S., Phillips, N. A., Bédirian, V., et al. (2005). The Montreal Cognitive Assessment (MoCA): a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
- Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). "Mini-mental state": a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. https://doi.org/10.1016/0022-3956(75)90026-6
- Morris, J. C. (1993). The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology, 43(11), 2412–2414. https://doi.org/10.1212/wnl.43.11.2412-a
- Cogstate. (n.d.). Using Cogstate digital assessments to identify cognitive impairment in Alzheimer’s disease clinical trials [White paper]. https://www.cogstate.com/fact-sheet/literature-spotlight-using-cogstate-computerized-tests-to-identify-cognitive-impairment-in-alzheimers-disease-clinical-trials/
Contact Us

