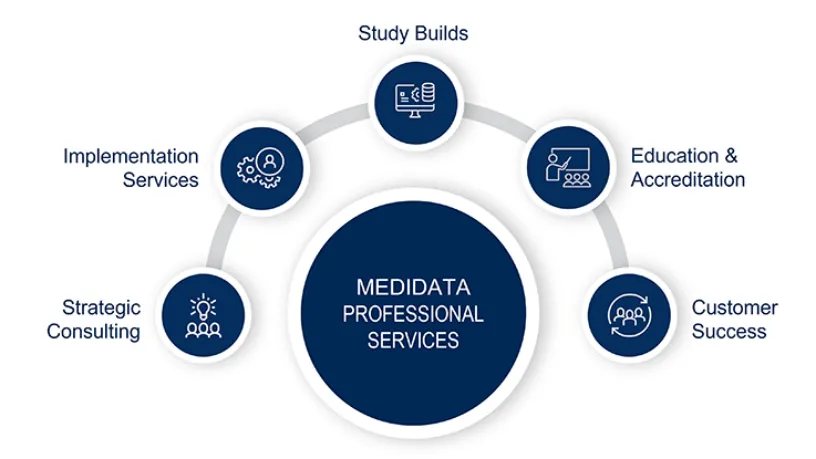
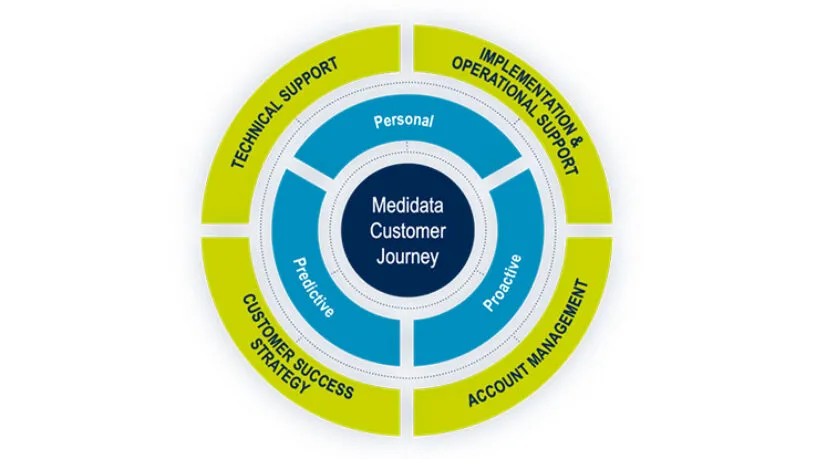
Medidata Global Education and Training
Expert Learning Solutions and Training to empower customers and partners in solving the impossible today!
Medidata global education and training enables clinical trial operation teams to expertly design, build and report on studies that bring life-saving therapies into the hands of waiting patients. Extensive, flexible, and secure.

Learning paths and training options for every clinical trial operations role.
Join millions of practitioners in building role-centric proficiency with Medidata products and solutions.
Explore Our Training Options

Course Catalog
Explore Medidata training courses!
We offer over 200 customer education units – self-paced eLearning courses that you can take anytime, and instructor-led courses that we deliver online and in-person (at our offices or yours).

Learning Paths
Quickly gain the knowledge you need to empower your clinical trial teams.
Our role-centric learning paths are designed to support professionals across the clinical trial operations industry. Gain the proficiency you need to build and conduct efficient, successful clinical trials.

Training Announcements
Stay on top of all training updates.
Ensure 100% of the team are informed about upcoming training updates and learning opportunities.

Pre-Scheduled Courses
Browse our pre-scheduled instructor-led course catalog to plan or advance your learning journey to success.

Certifications
Medidata professional certifications – empowering clinical researchers and clinical research administrators to learn and apply Medidata products and services, enabling smarter treatments and healthier patients.

Custom Training
Connect with your Medidata project manager to structure custom training that supports your unique needs, project timeline, or initiative.
Related Resources
How do I register for training?
Registering for pre-scheduled courses is easy using this link! Questions? Need Support? Just Contact Us!
Note: Medidata Global Education & Training courses are available to Medidata clients, partners and employees only.