Uncategorized
From eClinical to Patient-Centered mClinical
What role should patients play in clinical development going forward? Read More

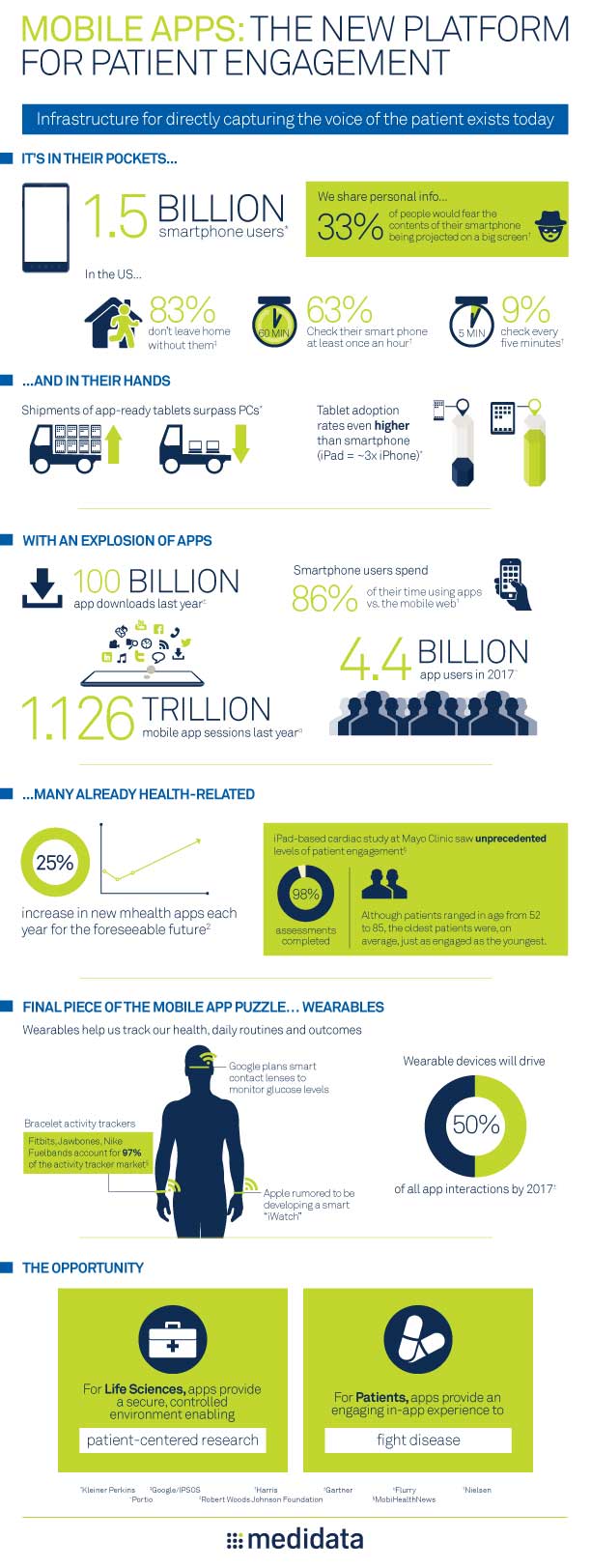
Mobile Apps: The New Platform for Patient Engagement
Capturing the voice of the patient has never been more critical and the life sciences industry now has the potential to harness the passion of patients to fight disease. Read More
An Easy Tool to Keep Patients Protocol-Focused
Isn’t it great when you get really good, helpful customer service? Read More

TransCelerate and Medidata Explore SDV Effectiveness in Upcoming Webinar
It’s no secret that clinical trial complexity and drug development costs are at an all-time high. With more than 30 percent of trial budgets allocated to site monitoring costs—and more than half of that being spent on source document verification (SDV) activities¹—the idea of implementing a risk-based monitoring (RBM) program to rid ourselves of the traditional 100-percent-SDV approach has the life sciences industry buzzing. Read More

How New Technologies Can Change the Way We Manage Clinical Trial Processes
We recently sat down with Robert Musterer, president of ER Squared, an eClinical consulting company partnering with Zifo Technologies. Robert presented at the Medidata Symposium on "Overcoming Inertia for Improved Clinical Trial Designs," a topic he covered in this recent Geeks Talk Clinical blog post. Read More
Challenges in Penetrating a Paper-Based Market with Technology-Based Solutions
The usage of electronic data capture (EDC) and other technology-based solutions in clinical trials—e.g., interactive web-response systems (IWRS), clinical trial management systems (CTMS) and electronic patient-reported outcome (ePRO) tools—has shown a substantial growth over the last decade. However in Israel, the adoption rate of EDC by locally-based companies has not increased, and many are still using paper-based solutions for their trials. Read More
