Medidata Secures a Leader Position in Everest Group’s PEAK Matrix® Assessment for eCOA, Driving the New Patient Experience Forward
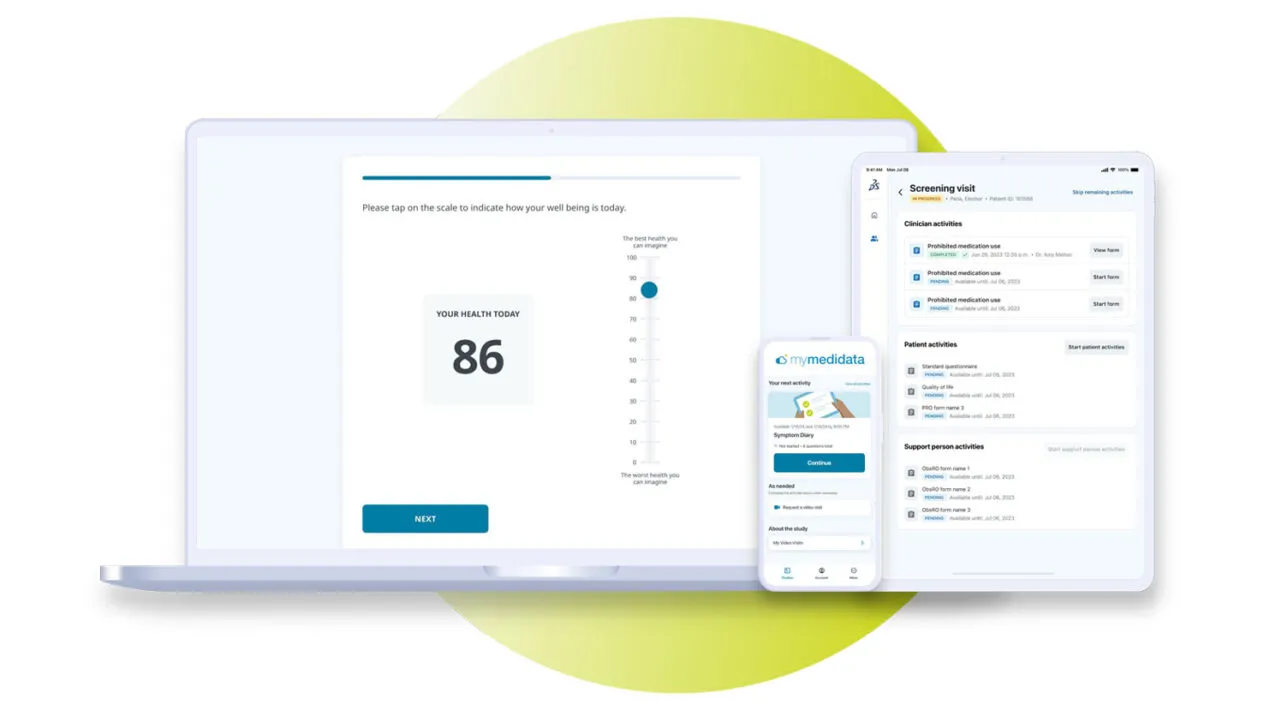
eCOA Solution – Built and Delivered with Speed and Quality
Centered Around the Patient Experience
Leverage a comprehensive eCOA clinical trial solution that cuts study build timelines, captures high-quality endpoints, provides real-time actionable insights, and puts those who matter most at the center of medical advancements – our patients.
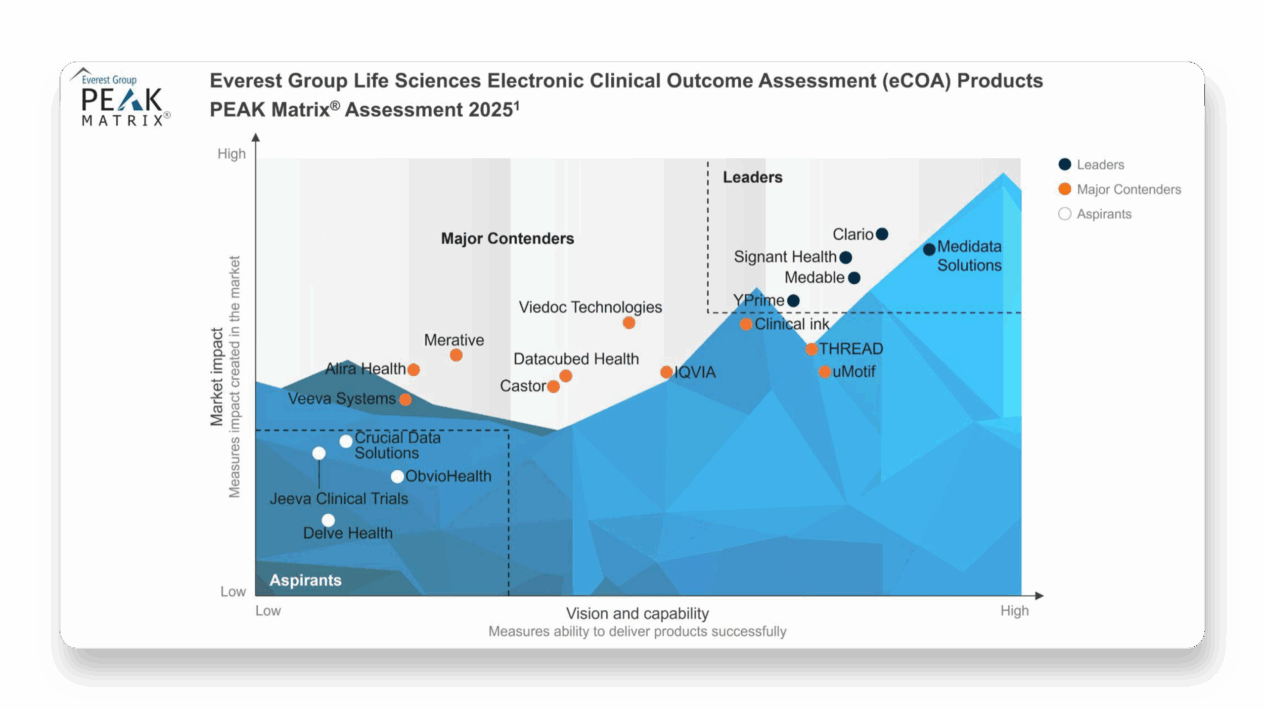
Recognized as the Highest Leader
Medidata eCOA secured the highest Leader position among the 19 providers assessed in the Everest Group’s PEAK Matrix® Assessment for eCOA 2025.
Industry-Leading eCOA Experience
Proven at scale with the expertise, global reach, and regulatory-ready eCOA solution to drive your clinical trial success.
Patients
Sites
Sponsors
Studies
Instruments
Language and Dialect Translations
Countries
Faster Study Build Time
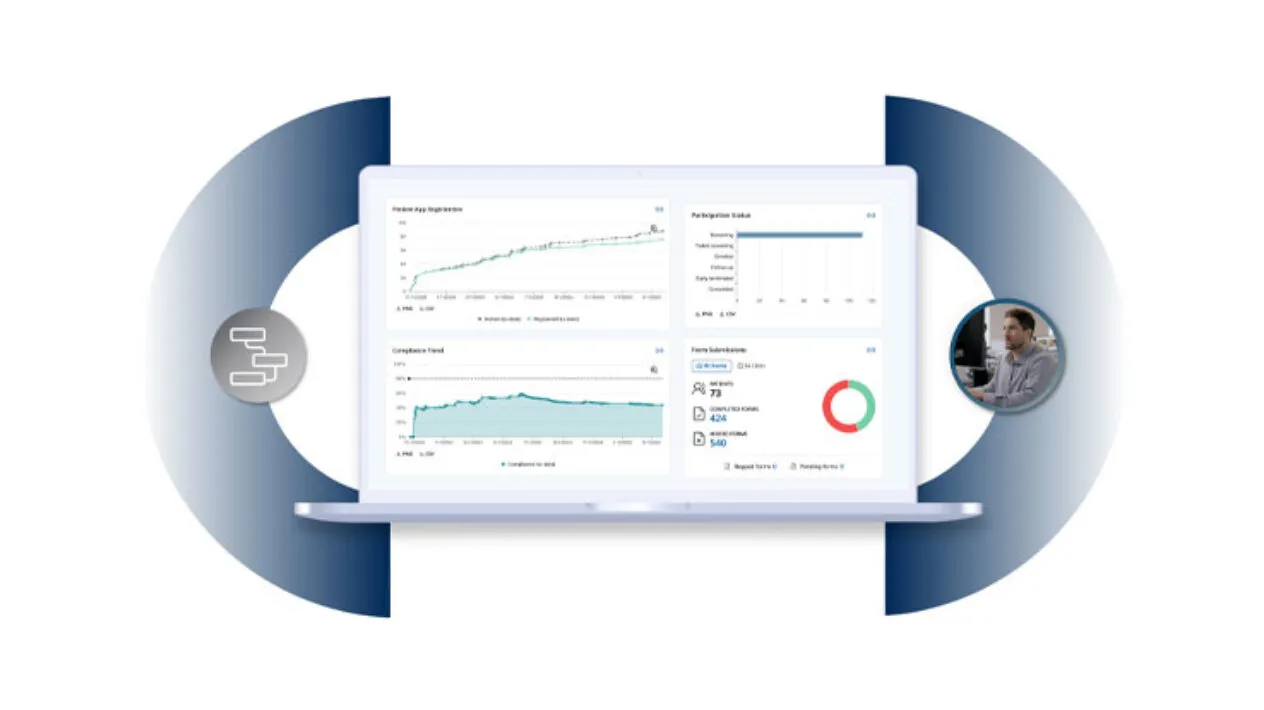
Gain Comprehensive, Real-time Insights
From patient-level compliance to trial-wide trends, sites and sponsors can gain instant visibility to monitor compliance, detect issues early, and take informed action.
From Build to Go-Live, Faster and Smarter, At Scale
Partner with Medidata’s eCOA Implementation Experts
Accelerated Study Build
Delivering a quicker study start time averaging 8 weeks compared to the industry standard of 12 weeks.
Scientific Expertise
Bringing our scientific expertise to the forefront with support for sponsors and partners in protocol development, guidance during study build, and more.
Global Device Support
Coordination and management of device provisioning lifecycle via our services team all the way through study close and device return.
Instrument Services
End-to-end licensing, translation, and localization support ensures your eCOA instruments are ready for global deployment with accuracy and compliance.
Data Services
The eCOA Data Services team provides leadership and support in order to uphold eCOA data accuracy.
24/7 Helpdesk
Elevated and localized support with a dedicated Patient Cloud Helpdesk for patients, sites, and sponsors, delivering efficient solutions and guidance with a 95% CSAT score.
Technology Adoption
Helping make the adoption of new technology seamless with a robust adoption plan, custom validation support, and Design Studios.
Unlock the full potential of your eCOA clinical trials
FAQ – eCOA Clinical Trials

Electronic Clinical Outcome Assessments (eCOA)
Clinical Outcome Assessments (COA) are instruments used to measure and capture how a patient feels and functions, typically administered as a questionnaire. eCOA involves the use of electronic devices such as provisioned mobile devices, tablets, web-based solutions, or patients’ own mobile devices (bring your own device, or BYOD) to collect patient data.
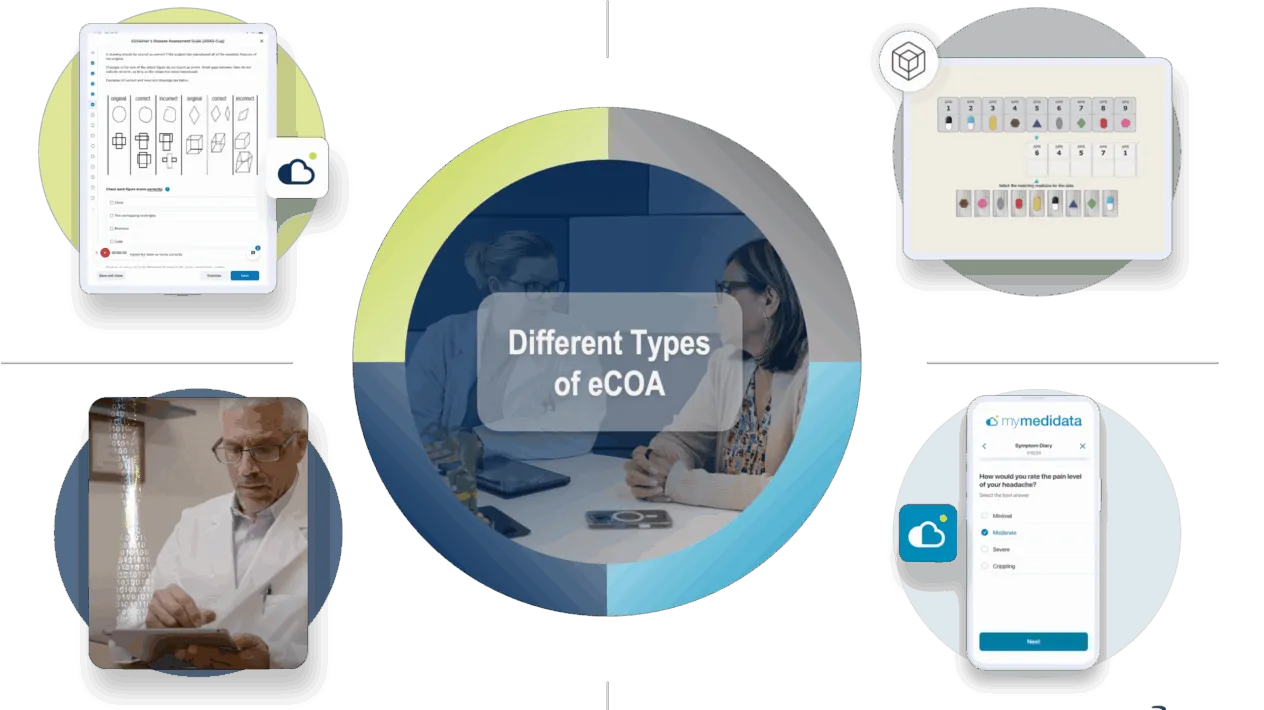
There are four major types of COA:
- Patient reported outcome (PRO)
- Clinician Reported Outcome (ClinRO)
- Observer Reported Outcome (ObsRO)
- Performance Outcome (PerfO)
When collected electronically, these are referred to as:
- Electronic Patient Reported Outcome (ePRO)
- Electronic Clinician Reported Outcome (eClinRO)
- Electronic Observer Reported Outcome (eObsRO)
- Electronic Performance Outcome (ePerfO)
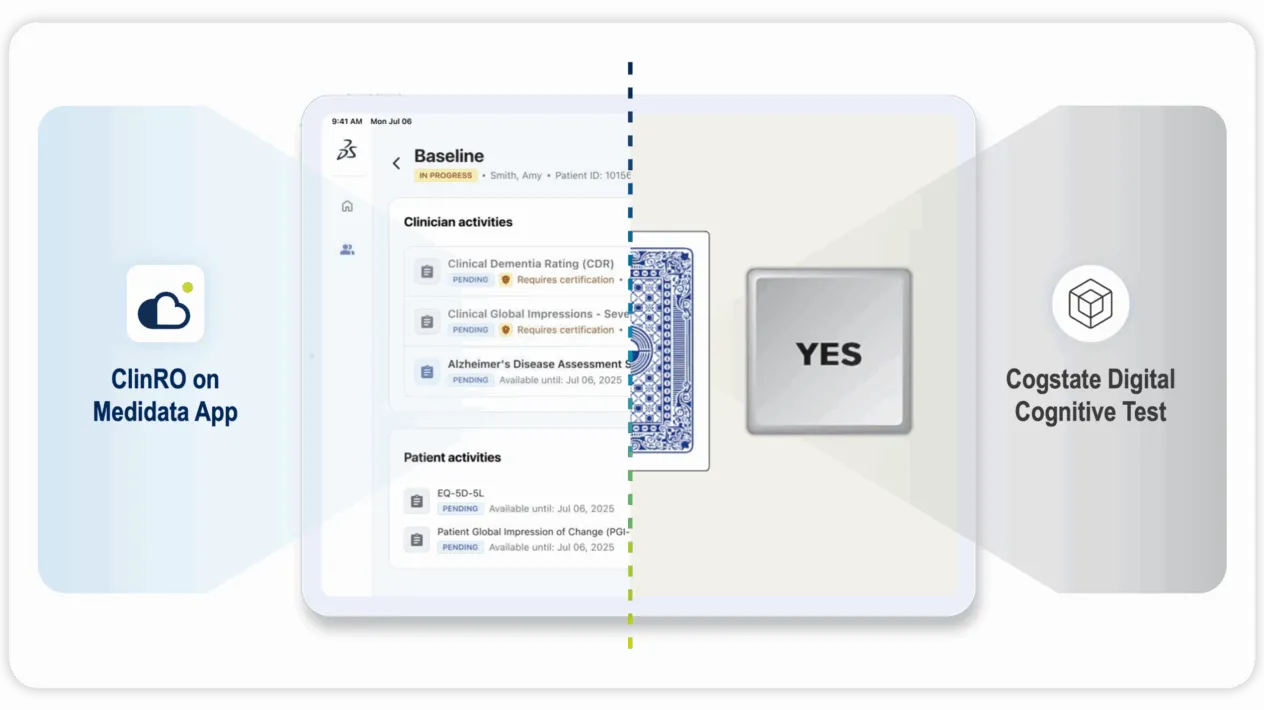
Yes, Medidata supports eCOA for complex studies like CNS trials.
Medidata and Cogstate’s partnership combines advanced system integration with robust technical, operational, and scientific expertise to provide comprehensive support for eCOA in CNS trials. This deeply integrated solution streamlines workflows and enhances endpoint data quality, empowering researchers to focus on driving meaningful outcomes in the complex CNS trial landscape.
Medidata collaborates with Patient Insights Board and Site Insights Board
Medidata actively collaborates with patients and sites throughout the development of its eCOA. Through dedicated boards, like the Patient Insights Board and Site Insights Board, we gather direct feedback on usability, burden, and workflow integration. This input helps shape both the software interface and the broader study experience to ensure that Medidata eCOA and the broader suite of solutions are both patient and site-centric by design and aligned with real-world needs.
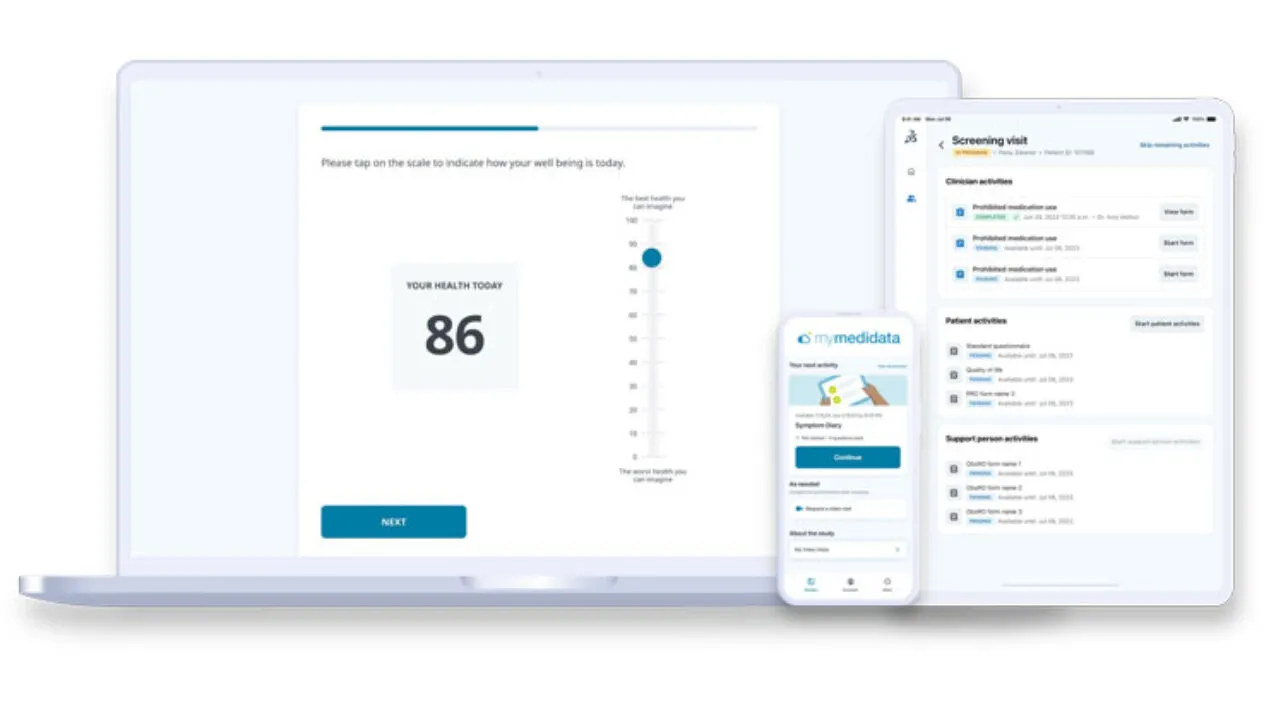
Yes, Medidata supports both provisioned device and BYOD
Medidata eCOA supports both provisioned devices and BYOD (iOS or Android), accessible via web or mobile app. This flexibility allows patients to complete assessments in the way that’s most convenient for them, whether at home, on site, or on-the-go, helping reduce burden, increase engagement, and improve compliance across diverse patient populations and geographies.