Medidata eCOA
As studies run, eCOA (electronic clinical outcome assessment) shouldn’t create rework, delays, or uncertainty in results.
Medidata eCOA supports faster study build and consistent capture of outcomes from patients, clinicians, and caregivers. It helps sponsors and CROs make real-time decisions based on high-quality eCOA data.
eCOA for Reliable Outcomes at Scale
Designed using real patient insight, Medidata eCOA balances the Patient Experience with scientific rigor, supporting all assessments from simple eDiaries to complex eClinROs.
Integrated Patient Experience
Site-ready Workflows
Faster Study Build with AI
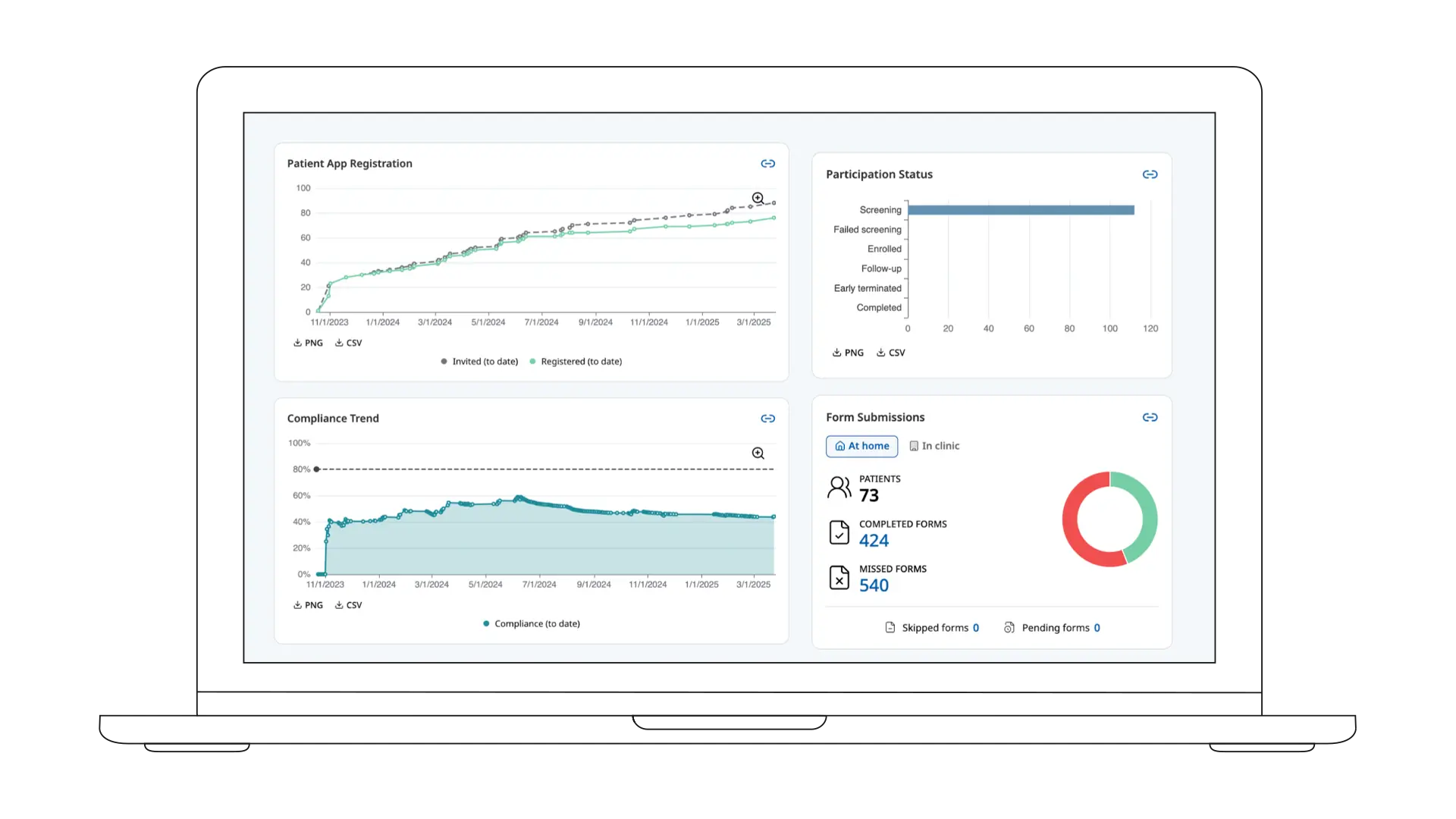
Comprehensive Real‑time Insights
Proven in Complex Studies
*Metrics reflect an average 24-month study and are for illustrative purposes only. Actual ROI for your trial will vary based on specific factors (e.g., sites, patients, duration).
What eCOA Delivers for Your Study
Patient Engagement
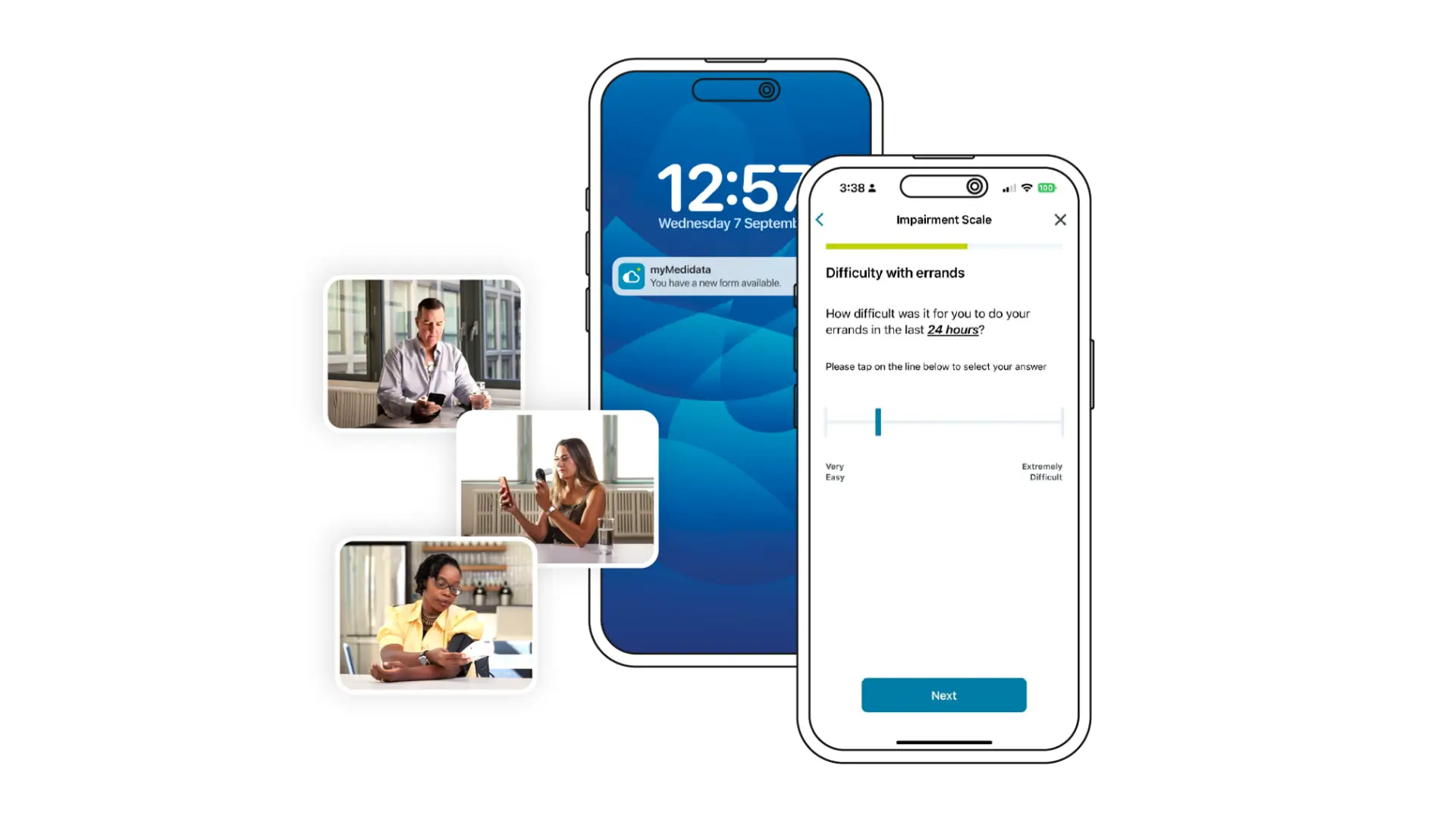
Single Experience for Patients
Patients complete Medidata eCOA through a single,
familiar experience that can also support consent, payments, and other study interactions.
Reducing fragmentation helps patients understand what’s expected and keeps them engaged over time for more complete outcome data.
Ready for a Better eCOA Experience?
Take our Readiness Survey to see how your current eCOA setup performs and where you can strengthen it. Takes less than three minutes.
Check Your eCOA ReadinessINDUSTRY RECOGNITION
Reduce Study Build Timelines by Up to 50%
Recognized as a Leader in Everest Group’s eCOA PEAK Matrix® Assessment 2025
Download Report

FAQ
Explore Experiences
Discover the Medidata Platform