Clinical Data Integration: Everything You Need to Know

Successful clinical trials rely on the collection and analysis of data from a wide variety of sources, including sites, patients, and labs, and linking clinical data to real-world data. Data collection typically comes from different systems and multiple vendors. Review and analysis are carried out by multiple stakeholders (data managers, CRAs, central monitors, medical monitors) at the CRO/sponsor, and that review/analysis can also involve multiple systems.
Clinical trial stakeholders are increasingly adopting complex research methodologies, such as adaptive trials, and implementing decentralized or hybrid studies. Combined with innovative technologies, these let them reap the benefits and opportunities presented by significantly increased volumes of valuable data and uncover deeper findings faster than before.
While new technology solves critical issues and can help research in countless ways, it often creates new challenges—most notably the difficulty of integrating data from many disparate sources. Communication across systems is crucial, but due to various factors, it’s far from seamless. The result is often negative, impacting the research itself and study participants.
In an ecosystem where collaboration is critical, effective clinical data integration and interoperability would address core issues of multi-system communication, unifying and empowering all stakeholders. So why is it such a challenge, what’s being done, and what does the future hold?
Read on to get insights into clinical data integration, data interoperability standards, and recommended best practices and tools.
What Is Clinical Data Integration in Clinical Research?
Clinical data integration can be defined as the process of aggregating and harmonizing clinical data from all available sources into a unified form for clinical trial stakeholders.
Data takes multiple forms—structured, e.g., case report forms (CRFs) and lab data, or semi-structured or unstructured, e.g., physician notes, freeform notes, and adverse event narratives.
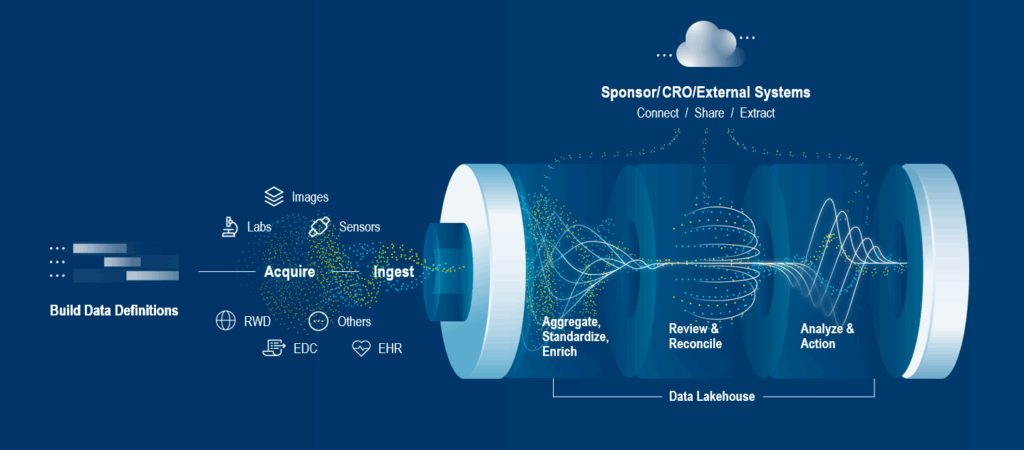
The most common sources of clinical research data are:
EDC (electronic data capture) systems: Originally, with the introduction of electronic case report forms, EDC systems replaced the pen-and-paper method for recording data. They have since evolved to become broader data acquisition and management systems. They capture site, patient, and lab-reported data, automating workflows and data reconciliation and providing valuable insights. Some EDCs also provide visibility of other data sources such as labs, eCOA, imaging summary data, and sensor summary data, and often include simple data review/monitoring and query capabilities.
ePROs (electronic patient-reported outcomes) and eCOAs (electronic clinical outcome assessments): Replacing traditional paper diaries and questionnaires, ePRO and eCOA solutions provide greater accuracy and patient engagement. This improves the patient experience while collecting higher-quality data and reduces errors in clinical trials.
Lab data (central and local labs): Many technological advances have been made in the highly complex management of patient samples, including laboratory information systems (LIMS), which record, manage, and store data for clinical purposes.
Imaging systems: Over 50% of clinical trials and 95% of all oncology trials use medical imaging, generating significant levels of data for analysis.1
Wearables and remote monitoring devices: The use of wearables, telemonitoring, and data tracking devices has seen tremendous growth. They’ve been proven to be an effective, non-intrusive, and patient-centric way of monitoring and collecting data.
EHRs (electronic health records) and EMRs (electronic medical records) for clinical and real-world data/evidence: A core source of clinical data comes from EMRs and EHRs, which include a patient’s medical history and other relevant information related to lifestyle, i.e., dietary patterns, smoking, work stress, etc. To put this into context, an average patient’s EHR is approximately 80Mb per year.2 In 2020, the World Economic Forum estimated that 2.3 zettabytes (2,300,000,000,000,000 megabytes of data) were produced within healthcare.3
Manual processes are still widely used, so data transfer from spreadsheets and documents also needs to be considered.
Why Clinical Data Integration Matters and Its Benefits
If clinical data is integrated into a centralized system, an appropriate technology platform can simplify and accelerate using AI and automation, reconcile and clean the data, and reduce manual effort for these tasks. It’s notable that EMR/EHR data for the same patient can differ from system to system, adding further complexity and the need for reconciliation.
Data integration from eCOA, ePRO, wearables, and telemonitoring sources is especially important as decentralized and hybrid studies are being implemented at an increasing rate.
Once the data has been cleaned and made research-ready, stakeholders are empowered with higher data quality and integrity and real-time data visibility, enabling faster decision-making and interim analysis. This reduces the time and cost of cleaning, enables faster study startup, and leads to faster database lock.
Protocol compliance is improved through centralized data surveillance and monitoring and the creation of standardized, traceable datasets, assisting regulatory compliance, submission, and audit readiness.
Clinical Data Integration Use Cases in Trials
Examples of how clinical trials benefit from clinical data integration include:
Clinical and real-world evidence integration: Integrating EHR and clinical data impacts the clinical research ecosystem. A few examples include enhancing the site experience by enabling faster completion of EDC forms, delivering deep and critically important patient insights for the study, and providing deep insights for external control arms.
Data reconciliation and cleaning: A highly time-consuming and error-prone manual task is transformed through data integration, advanced technologies like AI, and automation—ensuring consistent, clean data for interim and final analysis.
Safety monitoring: Comprehensive patient profiles can be used to identify potential safety/efficacy signals. Safety data management benefits from eliminating duplicate entries and most manual processes, reducing the need for adverse event reconciliation, query cycle times, and data review.
Site compliance: Data integration enables central monitoring to detect anomalies or outliers more effectively, enabling investigation and action at non-compliant sites.
Challenges in Clinical Trial Data Integration and Interoperability
The benefits of an integrated environment are compelling, but the industry still faces challenges in achieving a more effective and efficient interoperable environment for everyone.
Below are some key data integration challenges that have prevented complete integration and interoperability:
Data heterogeneity: There are data standards in place, but challenges exist—especially when unstructured data is collected, e.g., physicians’ notes. For example, the word ‘headache’ needs further context to be meaningful. Industry dictionaries are also updated at different intervals across vendors and sources, so regular reviews are needed during the study lifecycle.
Lack of interoperability: Clinical research systems (sponsor, CRO, site, and lab systems) use a multitude of eClinical systems that need to communicate with each other. There are still some interoperability challenges between healthcare systems (EHR/EMR) and clinical trial systems (EDCs).
Data standards and clinical trial/healthcare industry initiatives have both sought to address these challenges. Good progress has been made globally by governments and industry-backed programs.
Quality assurance: 100% source data verification (SDV) is one of the core methods used for quality assurance in a high proportion of studies today. It typically accounts for more than 50% of a trial’s resources and budget, but it’s recognized to be highly ineffective.4
Risk-based quality management (RBQM) methods and strategies take a targeted approach to data verification, and regulators actively encourage them. Clinical data integration plays an important part in achieving RBQM goals. Unfortunately, RBQM has seen slow adoption, but there has been progress in recent years.
Learn more about the role of SDV and SDR (source data review) in driving clinical trial data quality.
Sponsor–CRO–vendor coordination: Data transfers with inconsistent formats lead to manual checks and corrections, so the data is out of date by the time it’s ready for review.
With these challenges in mind, what is the industry doing to make clinical trial data integration more effective and efficient?
Core Standards and Models for Clinical Trial Data Integration
Globally, there are several data standards used to enable clinical data integration. Prominent among them are those developed by the Clinical Data Interchange Standards Consortium (CDISC). Their vision and mission are:
“… to amplify data’s impact and advance research by creating connected standards throughout the study information lifecycle, enabling data that is accessible, interoperable, and reusable for more meaningful and effective research.”
Their clinical data integration standards are categorized as foundational (the basis for defining data standards) or data exchange (the sharing of structured data across different information systems).5
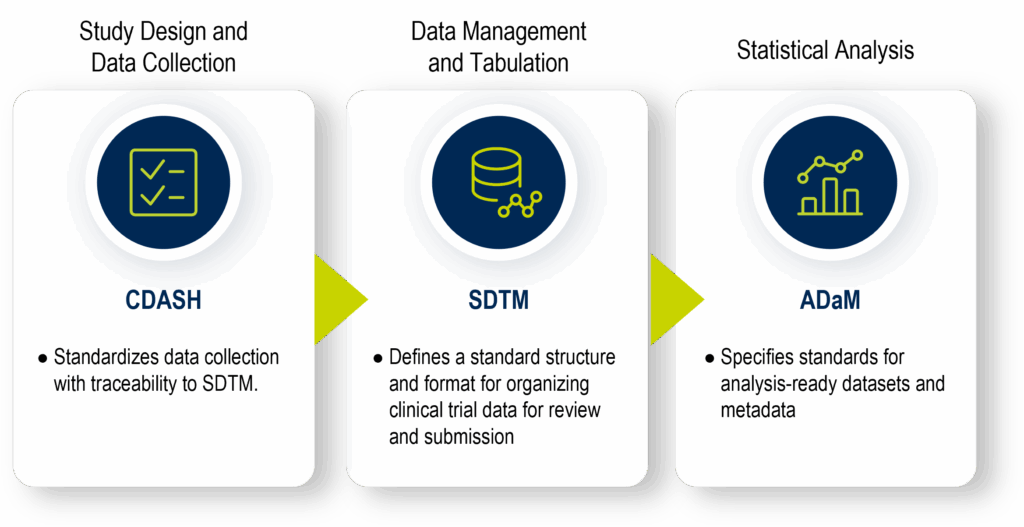
In summary, the foundational clinical data standards are:
- Clinical data acquisition standards harmonization (CDASH): establishes a standard way to collect clinical trial data consistently, using uniform CRFs, variable names, and metadata structures. Its purpose is to ensure clear traceability of collected data into SDTM, enhancing transparency and efficiency in data review and regulatory submissions.
- Study data tabulation model (SDTM): defines a standard structure and format for organizing clinical trial data into domains, facilitating data aggregation, management, analysis, reporting, and regulatory submission.
- Analysis data model (ADaM): specifies standards for analysis-ready datasets and metadata.
CDISC data exchange standards facilitate the sharing of structured data across different information systems. They are: Operational Data Model (ODM), Define-XML, Dataset-JSON, Dataset-XML, the Laboratory Data Model (LAB), Clinical Trial Registry (CTR)-XML, Study/Trial Design Model in XML (SDM-XML), and CDISC Standards in Resource Description Framework (RDF).
Health Level Seven International Fast Healthcare Interoperability Resources (HL7 FHIR) (2012) is also established as a set of resources and APIs to communicate healthcare concepts through standards-based clinical and healthcare data integration.6 It’s used for integrating EHR and real-world data.
Learn more about healthcare interoperability.
Best Practices for Implementing Clinical Data Integration into Trials
While efforts to create a global interoperability environment continue, there are current solutions that provide the best possible levels of clinical data integration available.
To leverage the benefits of an integrated data environment using existing tools and platforms, here are the best practices for implementing clinical data integration into studies:
- Define your integration goals for the trial early (e.g., eSource, EHR-EDC, decentralized capabilities).
- Map all data sources and their formats.
- Choose platforms that support open standards (CDISC, HL7 FHIR) and/or APIs.
- Align sponsors, CROs, and vendors on SOPs and formats.
- Validate and test data pipelines before launch.
- Establish a cross-functional integration governance team.
- Monitor integration performance and refine iteratively.
Tools and Platforms Supporting Data Integration in Research
With best practices in mind, (as multiple systems are reviewed as part of the assessment process), it’s clear that interoperability will be increasingly complex based on the number of diverse systems chosen. Industry experience has shown that complexity will go beyond data integration; it will include implementation, execution, resources, support, lead time, and cost challenges.
The proven path is to implement platforms that provide all the tools required without compromising on quality, efficiency, or speed, and to choose vendors with the relevant experience and resources to support the study.
Some basic clinical data integration capabilities to look for in a system are cross-vendor, multi-source data integration, standardization, and transformation tools, dashboards with real-time data, and advanced algorithms, artificial intelligence, and automation to help with data reconciliation and cleaning.
Current and Future Trends in Research Data Integration
Medidata understands the challenges faced in the industry, and its unified platform has always been designed and developed with clinical and healthcare data integration at its core. Medidata’s technology has a reputation for pushing the boundaries of what’s possible. Medidata Clinical Data Studio is the best-in-class solution for clinical data and quality management, interoperating with non-Medidata sources in both clinical trials and healthcare. It empowers stakeholders by leveraging advanced technologies, AI, and automation to streamline data aggregation, standardization, and management workflows.
Medidata already connects to a wide range of healthcare organizations and physician practices through national and local Health Information Exchanges (HIEs) and networks, and supports FHIR, Consolidated Clinical Document Architecture (CCDA), Digital Imaging and Communications in Medicine (DICOM), and HL7 standards.
The industry is also on the cusp of a new era of clinical data integration and technology.
Virtualization technology will redefine clinical trials. Medidata already provides Synthetic Control Arm® (SCA) and simulant (digital twins, simulated patients) technologies. AI-powered data cleaning and anomaly detection are part of Medidata’s next-generation architecture, and interoperability-as-a-service offerings are expanding rapidly.
Soon, human-in-the-loop AI virtual assistants will streamline trial processes, and in-silico clinical trials7 using real-world integrated patient data will be a reality.
The future of clinical trials is not far away, and clinical data integration is key to that next step.
Conclusion
Clinical data integration is not an option; it’s a critical requirement.
Data integration and interoperability must be a priority in every study. Existing data standards, advanced technology, planning, and experienced stakeholders are the enablers.
Following best practices and embracing integration plans early in the study design process limits risk and positions studies for success.
Once a global clinical data interoperability environment is fully formed, the resulting speed, efficiency, and results will be transformational for everyone involved.
The Medidata team has the experience to guide you through this process and the industry-leading unified clinical trial platform to empower you for success. Learn about Clinical Data Studio here or contact us to discuss your clinical trial data integration needs.
References:
-
- Clinical Trial Imaging
- Gopal, Gayatri, Suter-Crazzolara, Clemens, Toldo, Luca and Eberhardt, Werner. “Digital transformation in healthcare – architectures of present and future information technologies” Clinical Chemistry and Laboratory Medicine (CCLM), vol. 57, no. 3, 2019, pp. 328-335
- World Economic Forum (WEF), article for the WEF Annual Meeting, January 2024
- Hamidi M, Eisenstein EL, Garza MY, et al. Source Data Verification (SDV) Quality in Clinical Research: A Scoping Review. Journal of Clinical and Translational Science. Published online 2024:1-33. doi:10.1017/cts.2024.551
- CDISC Roadmap
- Health Level Seven Fast Healthcare Interoperability Resources
- An advanced multi-scale modeling and generative AI simulation run study.
Contact Us
