Trial Design & Synthetic Data Solutions
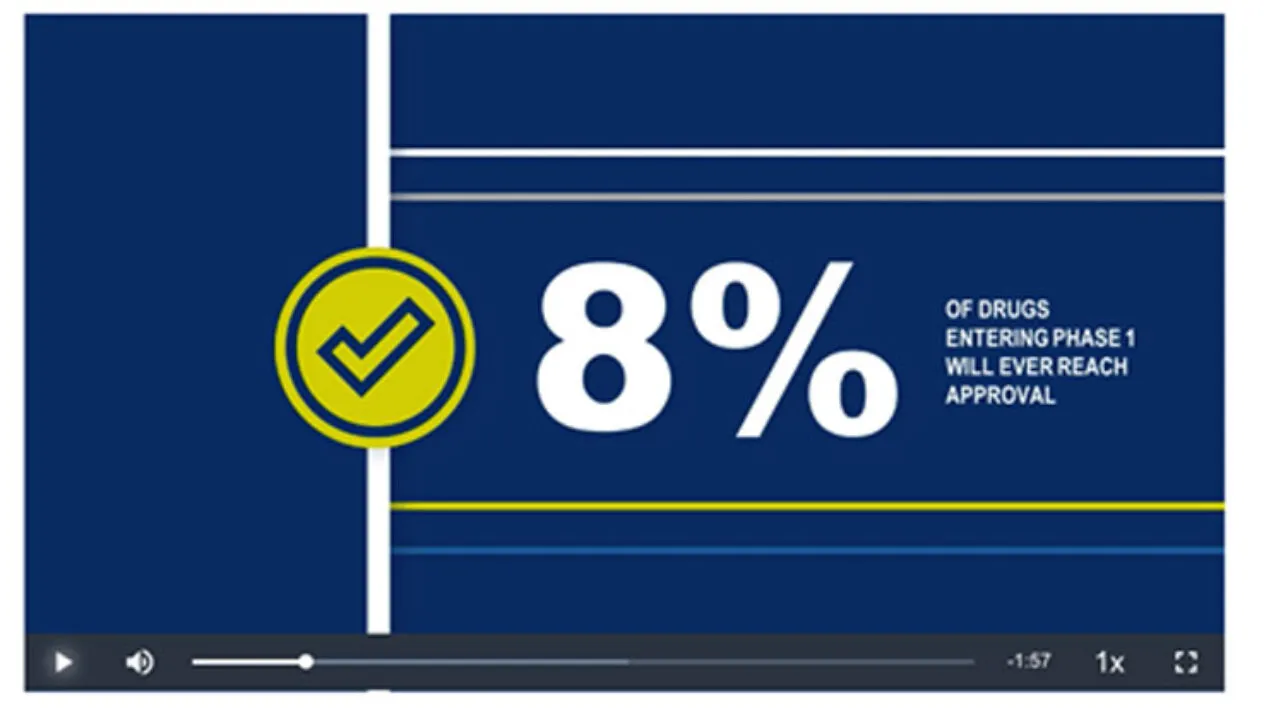
The race to develop therapies, obtain approval by regulators, and get new treatments to patients has never been more important, yet the obstacles along the way often present barriers to success. Medidata Trial Design offers solutions built off exclusive cross-industry global, historical clinical trial data comprised of 30,000 trials and 9 million patients.
Our team of industry experts partners with you to leverage this data to accelerate medical breakthroughs by delivering meaningful insights to create safer, effective clinical trial designs. We also offer Simulants, a pioneering synthetic data tool that delivers the insights contained within historical clinical trial data while safeguarding patient privacy and intellectual property.
Better data. Better insights.
Predict therapeutic efficacy
Unlock insights from clinical trial data to better understand your patient subpopulations and improve patient selection by analyzing unmet medical need under the existing standard of care and modeling inclusion/exclusion criteria to determine which specific subpopulations are most likely to respond to your therapy.
Compare consistency of outcomes under a standard of care over time to inform potential opportunities for label expansion.

Design safer trials
Monitor and benchmark patients throughout your trial to proactively determine which patients are the mostly likely to have an adverse event and mitigate and treat them proactively.
Medidata AI’s ground-breaking work in using historical clinical trial data to prevent CRS events in CAR-T trials has been accepted and presented at numerous industry conferences including ASCO, ASH and NCCN.

Prevent trial failures
Model trial scenarios to create the optimal protocol and predict obstacles before they become trial failures—enhancing your ability to identify patients who are likely to respond to treatment, inform dosing strategy or proactively mitigate serious adverse events. This translates to safer trials and an increased likelihood of successful therapeutic outcomes.
See our recently published work in The Journal of the Alzheimer’s Association which analyzed and identified factors that are predictive of participant dropout using pooled data from historical randomized clinical trials. These factors can be used to improve clinical trial protocol design as well as to develop prevention strategies during future trials.
Identifying predictive factors of patient dropout in Alzheimer’s disease clinical trials
Key Features

Powering a Clinical Data Revolution
Simulants is a groundbreaking synthetic data product – uniquely derived from Medidata’s exclusive repository of global, standardized historical clinical trial data, complete with covariates, endpoints, and variables as captured in their respective protocols. Simulants is optimized for generating high-fidelity synthetic data from clinical trial datasets, which contain uniquely sensitive data compared to other typical datasets used for synthetic data. Proven to meet rigorous regulatory and privacy constraints, Simulants empowers clinical developers with expanded data access, leading to increased research accuracy, insightful evidence, and optimized clinical trial design.

Exclusive historical clinical trial data
Leverage powerful AI modeling and generate evidence for your trial by connecting the data from over 30,000 cross-sponsored historical trials with more than 9 million patients from over 100 countries. This data comes complete with the traditional clinical trial style endpoints and complete covariate information, as they were designed in the clinical protocol, and captured, monitored and validated in the Medidata Rave electronic data capture (EDC) platform. This enables data-driven decisions by providing patient-level data in the common domains and over 100 harmonized variables.
“See examples of how our data has informed insights for sponsors of all sizes across multiple indications.”
Designing better trials with Generative AI
Medidata AI’s patented synthetic data generation models deliver superior quality and privacy preservation, outperforming leading synthetic data generators, including those from the MIT Synthetic Data Vault, in generating clinical data. Our innovative process, recognized with publications at conferences like NeurIPS and AMIA and a prestigious award from ICML, ensures the quality of synthetic data matches that of traditional clinical trial data. Unlike other methods dependent on large-scale training datasets, our approach excels regardless of limitations in clinical trial dataset sample size. This approach ultimately addresses patient privacy concerns, preserves data integrity, and ensures the highest level of research accuracy for teams to power their most important clinical decision-making.
Read our recent publication, “An interpretable data augmentation framework for improving generative modeling of synthetic clinical trial data” where we won the “Best Paper Award” at the Interpretable Machine Learning in Healthcare (IMLH) workshop at the International Conference on Machine Learning (ICML).

World-class expertise
Whether you are looking to bolster your findings to advance to the next phase of your study, understand the current unmet need under the existing standard of care or prevent adverse events – our highly qualified team of experts are your collaborative partners who work as an extension of your team. The Mediata AI team includes former members of the FDA, pharma and research community with regulatory, biostatistics, data science and medical oncology expertise.
Related Solutions
Our Research Alliance

About The Research Alliance
The Medidata Research Alliance was created to work with key clinicians and researchers from academia, industry leaders, and leading data scientists to conduct cutting-edge research to improve the field’s understanding of therapies, most notably in immunotherapies such as CAR-T and bispecifics:
Our historical clinical trial data is built upon over 20 years of approved and investigational products and can be leveraged to design and execute safer trials and advance scientific understanding. Our research topics commonly include:
- Understanding novel treatments that are associated with adverse events and benchmarks to mitigate or prevent catastrophic outcomes
- Refining clinical guidelines to improve outcomes and safety profiles for patients
- Expanding access of these therapies to patients who need it the most

CAR-T Research
Medidata AI offers the largest available database of CAR-T and Bi-specific T-cell Engagers (BiTEs) therapies, made up of over 70 cross-sponsor trials for approved or investigational therapies.
This data is not only used for commercial applications, including helping sponsors to design clinical trial protocols that help inform safety events or patient selection, but has been used by academics to show relationships between commonly collected lab data and the likelihood of adverse events.
Our work has been widely featured in peer-reviewed journals, conferences and the press:
ASCO 2022 Predictors of severe CRS in longitudinal CAR T-cell clinical trial data
ASH 2022 Predictors of severe CRS in longitudinal CAR T-cell clinical trial data
EHA 2023 Co-occurrence patterns of CRS and ICANS in patients undergoing autologous CD19-targeted CAR T-cell treatments
FierceBiotech Medidata links CAR-T’s cytokine release syndrome risk to common biomarkers in new study
CAR-T Cell Therapy Tanmay Jain speaks on CAR-T Cell Therapies and Medidata’s role in providing key insights.
CAR-T Trial Design Read this Whitepaper on enhancing CAR-T trial design with the Medidata AI CAR-T Data Cube.

Cardiovascular Research
Medidata has historical clinical trial data from 100,000+ patients that have been studied for MACE (Major Adverse Cardiovascular Events) such as stroke, myocardial infarction or heart attack, or heart failure. This includes patients who suffer from multiple comorbid conditions such as diabetes, obesity, hypertension, hyperlipidemia, and chronic kidney disease. Our data has a long follow-up time of up to six years, which is key to generating regulatory grade evidence. This dataset captures the most recent classes of drugs such as PCSK9, NOACs/DOACs, SGLT2, GLP1, and other standard-of-care therapies.
This data has been used in cutting-edge research including:
STH 2021 Comparing the Real-world and Clinical Trial Bleeding Rates Associated with Anticoagulation Treatment for Atrial Fibrillation
DDW 2022 Real-World Comparison of Healthcare Resource Utilization between Transoral Incisionless Fundoplication and Laparoscopic Nissen Fundoplication for the Treatment of Gastroesophageal Reflux Disease
DDW 2022 Real-World Comparison of Characteristics of Patients who Received Transoral Incisionless Fundoplication vs. Laparoscopic Nissen Fundoplication for the Treatment of Gastroesophageal Reflux Disease
ISPOR 2023: Validation of Novel Identification Algorithms for Nonfatal Myocardial Infarction Using Uniform Objective Criteria and Pooled Clinical Trial Data

Synthetic Data
Medidata offers anonymized patient-level data to investigate and inform product development. We bring together the power of our historical clinical trial database with cutting-edge AI/ML techniques that preserves patient privacy, while offering opportunities for data sharing, cross collaboration, and innovation for these valuable, siloed data sources.
Neurips 2022: A Source Data Privacy Framework for Synthetic Clinical Trial Data
Neurips 2022: Synthetic Clinical Trial Data while Preserving Subject-Level Privacy
_synthesize 2023: Synthetic Data Revolutionizing Clinical Trial Data Collaboration and Research