Digital Therapeutics (DTx): Everything You Need to Know

The pharmaceutical industry is at an inflection point. While traditional medicines have revolutionized modern healthcare by targeting the biological mechanisms of disease, they often focus on one dimension of patient care—the physical symptoms. This approach, while effective, could leave critical gaps in treatment around the behavioral and emotional components that underlie many chronic conditions.
Consider the reality: existing treatments may not effectively address the neurological foundations underlying many diseases, and patients have limited access to high-quality behavior-based treatments. Pharmacotherapy alone falls short of effectively treating the whole patient, creating an opportunity for innovative solutions to bridge this therapeutic gap.
Enter digital therapeutics (DTx)—a transformative category of evidence-based software designed to treat, manage, or prevent medical conditions. These aren’t simple health-tracking apps or wellness tools; they’re clinically validated, often FDA-cleared (through 510k or De Novo pathways) medical interventions delivered through software platforms.
Read on to discover how digital therapeutics are shaping the future of medicine. Learn what they are, how they work, who they benefit, where the industry is headed, and, most importantly for pharmaceutical companies: how to effectively integrate them into existing drug development and commercialization strategies.
Understanding the Digital Health Ecosystem
To understand digital therapeutics, it’s essential first to understand where DTx fits within the broader digital health landscape. Digital health serves as a catch-all term referring to the use of technology to create value across healthcare, encompassing a wide array of solutions with varying levels of clinical rigor and regulatory oversight.
The digital health ecosystem includes a diverse landscape of fitness and wellness apps, AI-enabled diagnostics, telemedicine platforms, companion apps, prescription digital therapeutics, and software-enhanced drugs. Products within this ecosystem require some degree of data security and validation, but a subset that are intended to identify or treat disease also require extensive clinical evidence and regulatory clearance. Business models range from direct-to-consumer offerings to partnerships with hospitals, employers, and payers.
Wellness Apps and Companion Apps: The Entry Point
Wellness apps (like Headspace or Noom) are software apps designed to work as standalone solutions to help patients manage their conditions. Companion apps (like Sidekick Health or Dario Health) are software apps that support the use of a pharmaceutical product by enhancing the patient experience, education, engagement, or adherence, but without delivering a therapeutic intervention themselves. They’re “companions” to the drug—not substitutes or therapies.
Neither type of application delivers clinical benefits and both have limited regulatory oversight from the FDA. Wellness apps’ benefit claims are typically under the oversight of the Federal Trade Commission (FTC), while the companion apps’ benefit claims are typically restricted to promotional materials regulated by the Office of Prescription Drug Promotion (OPDP) and generally cannot be added to drug labels.
These applications typically provide patients with supportive tools and features such as medication reminders, dosing instructions or tracking, educational content, and symptom tracking. They may sometimes include wearable integration or communication with care teams.
Digital Therapeutics: Evidence-based Interventions
Digital therapeutics represent a distinct subset of digital health focused on delivering therapeutic interventions via software. According to the Digital Therapeutics Alliance, DTx are defined as “health software intended to treat or alleviate a disease, disorder, condition, or injury by generating and delivering a medical intervention that has a demonstrable positive therapeutic impact on a patient’s health.”1
These products leverage established behavioral science principles, incorporating evidence-based approaches like cognitive behavioral therapy and dialectical behavior therapy. They may be further enhanced through gamification principles that boost patient adherence and AI-powered personalization engines that can adapt treatment protocols to individual patient needs and responses.
Digital therapeutics are recognized as medical devices and are subject to internationally recognized standards and national regulations. These software solutions undergo regulatory review similar to traditional medical devices, albeit with different clinical evidence requirements.
Several core characteristics distinguish digital therapeutics:
Evidence-based: DTx interventions are grounded in clinical research and validated therapeutic approaches, often incorporating established methodologies like cognitive behavioral therapy (CBT) or dialectical behavior therapy (DBT).
Clinically evaluated: DTx interventions undergo clinical evaluation (typically through randomized controlled trials) to demonstrate safety and efficacy before regulatory approval.
Regulated: Most digital therapeutics are cleared or certified by regulatory bodies like the Food and Drug Administration (FDA) in the U.S., European Medicines Agency (EMA), and Pharmaceutical and Medical Devices Agency (PMDA) in Japan, to make sure they meet stringent safety and efficacy standards.
Integrated: DTx interventions integrate with care providers and health system workflows, enabling healthcare professionals to prescribe and monitor these interventions as part of comprehensive treatment plans.
Core Principles of Digital Therapeutics
The Digital Therapeutics Alliance has established core principles that guide DTx development:
- Incorporate design, manufacturing, and quality best practices
- Engage end users in product development and usability processes
- Incorporate patient privacy and security protections
- Apply product deployment, management, and maintenance best practices
- Publish trial results, inclusive of clinically meaningful outcomes, in peer-reviewed journals
- Be reviewed and cleared or certified by regulatory bodies as required to support product claims
- Make claims appropriate to clinical evaluation and regulatory status
- Collect, analyze, and apply real-world evidence and product performance data
Categories and Applications
Digital therapeutics are generally classified into three primary categories: treating a disease, managing a disease, or improving a health function. Common delivery modalities include smartphone apps, wearables, connected devices, and web platforms.
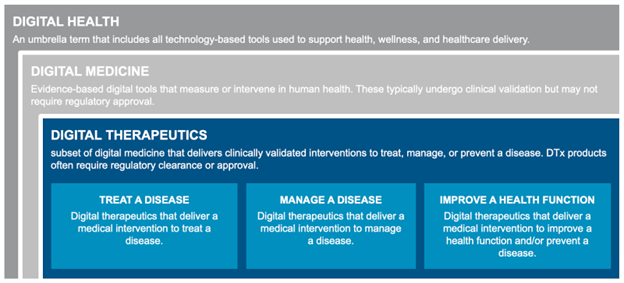
DTx currently address a wide range of conditions, including ADHD, depression, diabetes, hypertension, substance use disorder, insomnia, irritable bowel syndrome, chronic pain, and migraines, with new therapeutic areas continuously emerging.
Clinical Workflow Integration
The clinical workflow for digital therapeutics varies depending on the specific product and regulatory status. Some DTx require a prescription from healthcare providers, while others may be accessed directly by patients, depending on the country and specific product approval.
Patients will typically engage with DTx for weeks to months, and usage data and treatment progress are shared with care teams or insurance providers for monitoring and outcome assessment. This continuous data flow enables real-time treatment adjustments and supports evidence-based clinical decision-making.
Delivery Mechanisms and Integration
Digital therapeutics are designed with mobile-first interfaces and should be built to integrate seamlessly with existing digital health ecosystems. Many DTx platforms can connect with wearables and sensors to collect biometric data, such as heart rate, sleep patterns, and glucose levels, creating comprehensive patient health profiles.
DTx can function independently or integrate with other interventions, including digital health technology components for monitoring, diagnostics, clinical decision support, and tandem medical interventions like clinician-delivered therapies, pharmaceuticals, and medical devices.
Prescription Digital Therapeutics: The Regulated Standard
Prescription digital therapeutics (PDT) represent the gold standard within the DTx category and are regulated as medical devices. These software-based interventions are delivered through mobile apps or hardware devices (such as virtual reality) that make treatment claims and are designed to deliver clinical outcomes through therapeutic interventions like cognitive behavioral therapy, mindfulness training, and health behavior change.
PDTs are characterized by their intentionally designed treatment regimen—typically requiring only a few minutes of daily engagement to deliver the desired clinical outcome. They’re clinically validated through randomized controlled trials and cleared as Software as a Medical Device (SaMD), requiring prescription by licensed healthcare providers.
PDTs can function as standalone treatments (prescribed independently) or as complementary therapies (adjunct to pharmacotherapy), with both pathways utilizing 510(K) or De Novo regulatory clearances without requiring drug label expansion.
Software-enhanced DrugsTM: The Future of Combination Therapy
Software-enhanced Drugs (SE Drugs) are an innovative approach combining digital therapeutics with specific pharmaceuticals in a single prescription. This drug-software combination therapy delivers measurable clinical benefits compared to the drug alone. This approach has been supported by the FDA’s Prescription Drug Use-Related Software (PDURS) draft guidance, which provides the regulatory framework for integrating therapeutic software with pharmaceutical products.
The PDURS draft guidance clarifies how the combination of prescription digital therapeutics with specific drugs can be co-branded and integrated into a single prescription. This regulatory pathway provides a unified efficacy and safety signal that has its own clinical claims, beyond what the drug alone can achieve. It demonstrates that the FDA is looking ahead and recognizing the opportunity for software to add meaningful clinical benefit to existing and future pharmaceuticals.
SE Drugs are co-packaged drug-software combination products with their own unique National drug code (NDC), similar to drug formulations like extended-release versus immediate-release versions.
This approach allows physicians to prescribe the combination as a unique therapy option, effectively creating new digital formulations of existing or novel drugs—available to providers and patients much like an ‘XR’ or extended-release version of a drug, but as an ‘SE’ software-enhanced formulation.
The software component of SE Drugs is built as Software as a Medical Device (SaMD), meeting FDA quality, cybersecurity, and regulatory standards, while the combined product goes through drug regulatory approval pathways. This model promises to offer unique benefits in an easy-to-access co-packaged formulation, leveraging existing drug reimbursement pathways, integrating digital benefits into existing prescribing workflows, and representing how medicines are already prescribed today.
Want to hear how Medidata and Click Therapeutics are driving the expansion of SE Drugs? Watch our recent webinar “Patients, Pipeline & Promise: Software-enhanced Drugs”
Strategic Development Pathways for Pharmaceutical Companies
For pharmaceutical companies considering developing a drug-software combination product, timing and strategic approach are critical factors that significantly impact cost, complexity, and regulatory requirements.
- Early Development Integration (phase I/II): Integrating software during phase I or II trials represents the most straightforward and strategic entry point. These exploratory phases offer flexibility for adding software-enhanced elements with minimal cost while helping sponsors de-risk safety concerns. For example, in dose-ranging studies involving drugs with potential toxicity, software interventions can help manage side effects or improve adherence, potentially improving safety and tolerability outcomes.
- Market-ready Drugs: For approved drugs already on the market, obtaining FDA label updates with software components offers a fast and cost-effective pathway. Trials using synthetic control arms can demonstrate the added clinical benefit of drug-software combinations versus the drug alone, significantly reducing the need for large control groups and associated site costs. Alternatively, sponsors may launch companion apps first to generate real-world evidence for a subsequent FDA submission when ready for SaMD clearance.
- Phase III Considerations: Incorporating software at phase III represents the most complex scenario due to high stakes and regulatory sensitivity. Sponsors may be reluctant to risk pivotal study integrity by introducing new variables during the most expensive and sensitive trial phase. The payoff is a differentiated therapy with richer evidence and a stronger commercial potential. Careful consideration should be taken to ensure the added component demonstrates clear value with minimal risk and an aligned strategy.
Benefits of Enhancing Drugs Using Digital Therapies
Enhanced Clinical Outcomes
Digital therapeutics offer new levels of scalability, providing therapeutic interventions remotely—anytime, anywhere. This accessibility is particularly impactful in rural or underserved regions where specialist care may be limited or unavailable. For pharmaceutical companies, this means expanded market reach and the ability to serve patient populations previously difficult to access.
In addition to the benefit of broad access, digital therapeutics continue to demonstrate measurable improvements in clinical outcomes compared to standard care alone. Clinical studies are showing significant patient benefits, such as reductions in symptom frequency and severity. For instance, in its pivotal study involving over 500 patients, Click Therapeutics’ CT-132 demonstrated a mean reduction of 3 monthly migraine days over a 12-week period in the active treatment group (compared to the sham control group2*).3
These improved outcomes can translate to reduced hospitalizations, fewer disease relapses, and enhanced overall patient quality of life—metrics that are increasingly important to payers and healthcare systems focused on value-based care.
Cost-Effectiveness and Reimbursement Advantages
Prescription digital therapeutics (PDTs) have traditionally struggled to achieve drug-like reimbursement due to the need for digital access infrastructure and uncertainties around how they fit into the benefit category classification. Health plans use this classification to categorize prescription medications and determine the associated cost-sharing for patients, typically achieved through a formulary or prescription drug list. This classification gets complicated for those PDTs using an intent-to-treat approach, in which outcomes are analyzed for all patients who were prescribed or assigned to use the digital therapeutic—whether they used the software as intended, dropped out, or deviated from the protocol.
By combining clinically driven PDTs with drugs, the resulting combination products offer simplified reimbursement pathways and greater pricing flexibility. These products follow familiar formulary placement and coverage determination processes through existing pharmacy and therapeutics (P&T) committees at health plans. Additionally, the use of a dedicated national drug code (NDC) resolves longstanding coding and billing challenges faced by standalone SaMD, enabling seamless back-end processing of product distribution.
This model lets physicians prescribe these products through existing electronic medical record (EMR) systems and may extend the lifetime value of the original drug by transitioning patients to the drug-software combination as the product approaches the end of its patent life.
Patient Empowerment and Engagement
Digital therapeutics enable active patient participation in treatment through real-time feedback and engagement mechanisms. These products can deliver personalized treatments tailored to individual patients, aligning with the broader trend of real-world outcomes and patient empowerment. Patients become partners in their care journey rather than passive treatment recipients, leading to improved adherence and better long-term outcomes.
Improved Adherence through Behavioral Interventions
Medication adherence remains a persistent challenge in healthcare—medicines don’t work if patients don’t take them. Digital therapeutics can fill this adherence gap through notifications, gamification, and behavioral nudges that keep patients on track. The software component can improve patient outcomes while increasing the likelihood of drug adherence, creating a synergistic effect that benefits both patient health and commercial success.
Regulatory Landscape: Navigating Approval Pathways
PDURS: An FDA Framework for Regulating Software Associated with Prescription Drugs
The FDA’s Prescription Drug Use-Related Software (PDURS) draft guidance provides a regulatory framework for associating software with pharmaceutical products. To qualify as PDURS, software must be “disseminated by or on behalf of the drug sponsor” and provide “output that supplements, explains, or is otherwise textually related to a sponsor’s drug.”4
The labeling must clearly distinguish between Required and Optional software:
- Added Clinical Benefit: Software + drug demonstrates evidence of added clinical benefit compared to the drug alone. This criterion offers opportunities for existing and future drugs to digitally upgrade their outcomes with optional therapeutic software.
- Required for Safe and Effective Use: Software is required for safe and effective use of the drug and must be used to achieve the intended effect.
Meeting these criteria enables inclusion in FDA-required labeling and promotional materials. If the requirements are not met, claims for the PDURS combination product are limited to promotional labeling only.
Global Regulatory Bodies and Approval Pathways
Digital therapeutics are regulated by major agencies including the FDA (U.S.), EMA (Europe), MHRA (UK), and PMDA (Japan). The FDA’s De Novo pathway provides a route for novel software devices, while most DTx are classified as class II medical devices.
Evidence requirements include clinical trials (with randomized controlled trials preferred), comprehensive safety and efficacy data, and post-market surveillance through continuous data collection and updates. Regulatory pathways currently exist for PDURS engagement, beginning with identifying treatment gaps that software can address and building target product profiles for software-enhanced drug formulations.
Different regions offer varying regulatory approaches. Germany's DiGA Fast-Track system provides accelerated approval pathways, the UK’s NICE framework offers structured digital health evaluation, and Japan shows growing acceptance of SaMD approvals.
The Digital Therapeutics Alliance—a non-profit trade association of industry leaders and stakeholders engaged in the evidence-driven advancement of digital therapeutics—serves as a unified industry voice providing guidance and advocacy for regulatory advancement and helping shape policies that support DTx development and adoption.
Emerging Trends and Future Outlook
The digital therapeutics landscape will continue to evolve with AI-based treatment personalization, integration with smartwatches and virtual reality platforms, and the use of digital biomarkers for early disease detection. These advances promise more sophisticated, personalized interventions that adapt to individual patient needs and preferences.
The global market is projected to reach $21.9 billion by 2028, driven by increased venture capital investment and growing partnerships between DTx companies and pharmaceutical manufacturers5.
This growth reflects recognition of DTx’s potential to transform healthcare delivery and improve patient outcomes. Further market expansion is also possible when all pharmaceutical manufacturers start to add software to their drug assets.
Expanding Therapeutic Areas
Digital therapeutics are expanding into more therapeutic areas, driven by growing clinical evidence and regulatory acceptance. This expansion reflects DTx technology’s maturation and the pharmaceutical industry’s recognition of software’s potential to address previously unmet clinical needs.
We’ll continue to see digital therapeutics move beyond their initial focus areas of behavioral health and chronic disease management into more complex therapeutic domains. This expansion is characterized by increased clinical rigor, more sophisticated regulatory submissions, and growing pharmaceutical industry partnerships. The trend is indicative of a shift from standalone DTx solutions toward integrated drug-software combinations that leverage the PDURS framework for enhanced clinical outcomes. Key therapeutic areas to watch include:
Mental Health: The Leading Edge
Mental health represents the most advanced DTx expansion area, with applications for PTSD, anxiety disorders, depression, and substance use disorders showing particularly strong clinical evidence. These conditions are well-suited for software-enhanced approaches because they have underlying faulty neurological mechanisms and require specialized behavioral interventions that traditional pharmaceuticals alone cannot provide. Software-enhanced drugs in this space could combine anxiolytics or antidepressants with cognitive behavioral therapy platforms, potentially improving both efficacy and adherence while reducing side effects.
Oncology Support: Addressing Treatment Complexity
Oncology represents a significant opportunity for software-enhanced drugs, especially in treatment adherence, symptom management, and quality of life improvement. Cancer treatments often involve complex regimens with significant side effects, making them ideal candidates for software enhancement. Future applications include chemotherapy drugs paired with symptom management software or immunotherapy treatments combined with platforms that help patients recognize and report adverse events in real time.
Special Applications: Filling Critical Gaps
Digital therapeutic solutions address historically underserved populations where traditional clinical trials face unique challenges, such as pediatrics and pregnant populations—both of which may benefit from side-effect-free digital intervention.
- Pediatrics: Software-enhanced drugs could revolutionize treatment for ADHD, autism spectrum disorders, and childhood anxiety by combining lower-dose medications with engaging, age-appropriate behavioral interventions delivered through gaming platforms and interactive applications.
- Pregnant populations: These solutions can offer personalized, scalable, and non-pharmacological interventions, making them particularly appealing when drug use is limited due to fetal safety concerns.
Other Areas to Watch for Software-enhanced Drugs
Several therapeutic areas show promise for software-enhanced drug development under the PDURS framework:
Neurological Disorders: Conditions like migraine, epilepsy, and multiple sclerosis could benefit from drugs paired with trigger identification software, seizure prediction algorithms, or mobility tracking platforms that optimize treatment timing and dosing.
Metabolic Diseases: Diabetes and obesity treatments represent prime candidates for software enhancement, with medications combined with continuous glucose monitoring integration, dietary coaching platforms, and exercise optimization algorithms.
Cardiovascular Health: Hypertension and heart failure medications could be enhanced with software that provides real-time blood pressure monitoring, medication timing optimization, and lifestyle modification support.
Addiction Medicine: Substance use disorder treatments show significant potential for software enhancement, combining pharmacotherapy with behavioral support platforms, craving management tools, and relapse prevention systems.
Pain Management: Chronic pain represents a complex therapeutic challenge where software-enhanced drugs could combine analgesics with cognitive behavioral therapy platforms, activity tracking, and personalized pain management strategies.
Each of these therapeutic areas represents opportunities for pharmaceutical companies to enhance existing portfolios and potential entry points into new markets. Software-enhanced approaches could establish new standards of care and create competitive advantages through improved patient outcomes and differentiated value propositions.
The Path Forward: Digital as the Future of Medicine
Digital therapeutics represent a shift in healthcare delivery, offering accessible, evidence-based, personalized interventions that complement and enhance traditional pharmaceutical treatments. As the industry evolves, DTx and software-enhanced drugs will become integral to chronic disease management, mental health care, and overall wellness strategies.
“The app shows clinical benefit, the drug shows clinical benefit, but the real vision is that these two things in combination are better than either one independently. So you're really getting a true 1 + 1 = 3 benefit in the label.”
– Anthony Costello, CEO Medidata
For pharmaceutical companies, the question isn’t whether to engage with digitally enhanced therapies, but how quickly and strategically to integrate these solutions into their development pipelines and commercial portfolios. Companies that recognize digital as the future of medicine and act accordingly will be best positioned to serve patients and succeed in an increasingly competitive healthcare marketplace.
The convergence of therapeutic expertise with scalable technology empowers sponsors to develop a new generation of treatments that are more engaging, more effective, and better aligned with patients' needs. By uniting deep therapeutic experience with innovative digital solutions, the pharmaceutical industry can drive forward treatments that truly transform patient care and outcomes.
Medidata and Click Therapeutics believe in the future of digital medicine. To explore how software-based treatments and software-enhanced (SE) Drugs can help elevate your portfolio and accelerate your pipeline, visit www.medidata.com/click.
Together, we can lead the healthcare industry towards better outcomes.
References
- https://dtxalliance.org/understanding-dtx/what-is-a-dtx/
- A sham control group is a comparator group that receives a placebo-like intervention designed to mimic the active treatment—including its look, feel, and delivery—but without the therapeutic component.
- https://www.medscape.com/viewarticle/first-class-prescription-digital-therapeutic-effective-2025a10008h6?form=fpf
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/regulatory-considerations-prescription-drug-use-related-software
- https://www.researchandmarkets.com/reports/5390138/digital-therapeutics-dtx-market-by-offerings
Contact Us

