Medidata Remote Source Review
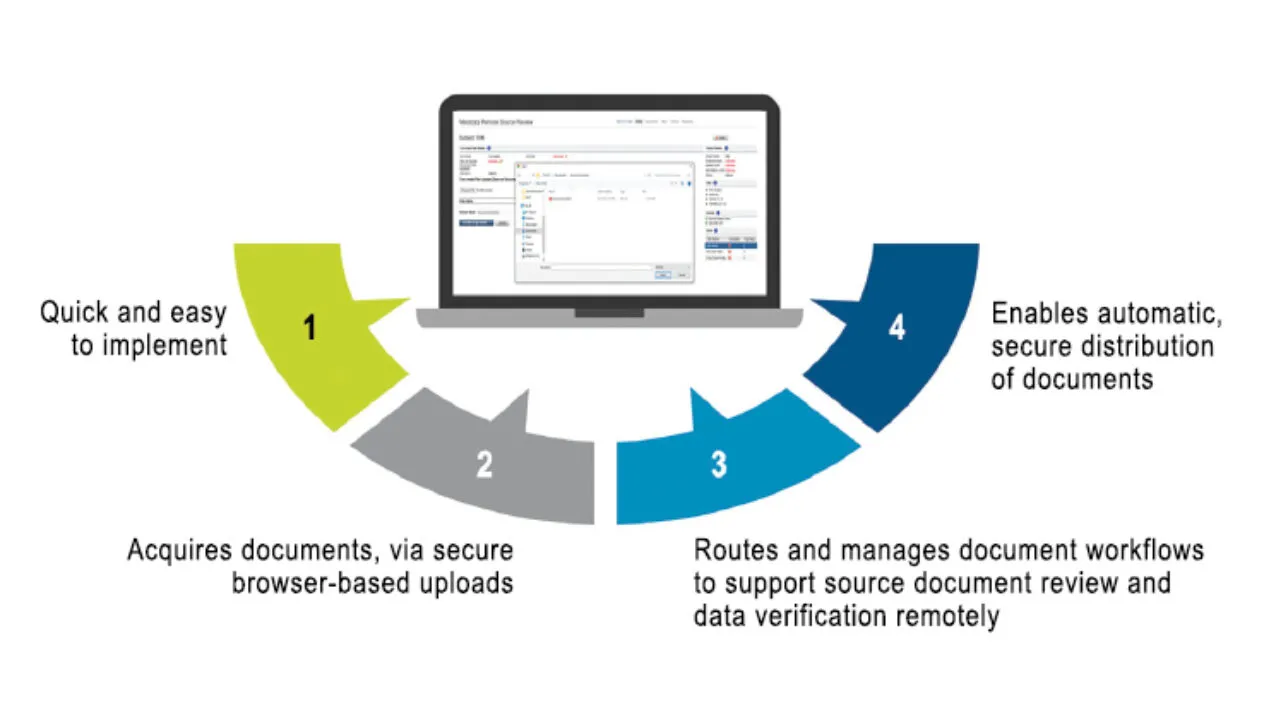
Success in clinical trial operations now requires a dedicated remote site access and monitoring strategy. Medidata Remote Source Review, part of the Medidata Data Experience, is a cloud-based solution that rapidly and remotely enables your monitors to acquire critical documents, automates document workflows to the right monitor for the right study and site, and allows them to review documents to support Source Data Verification (SDV) and Source Data Review (SDR) for compliance and quality.
Remote Source Review is a critical component of remote monitoring, a holistic, innovative digital solution to enable a flexible on-site/off-site approach to study oversight and optimize your Risk-Based Quality Management strategy.
Optimize Remote Clinical Trials with Medidata Remote Source Review
Remote Monitoring in Clinical Trials Saves Costs
Remote Monitoring of critical source documents reduces the costs of your monitors prepping for and going on-site for SDV/SDR, eliminating site travel and per diem costs for those visits.

Reduce Clinical Trial Site Burden
With simple upload and redaction tools, and automated workflow/task management, sites need less time to prepare for monitoring visits for SDV/SDR. Remote Source Review can be connected to Rave EDC which automatically creates study subjects, reducing time spent by site personnel entering data. Remote Source Review is part of the Medidata Platform and users benefit from a single sign-on through iMedidata.

Improve Clinical Trial Study Efficiency and Speed
Since off-site/remote monitoring removes monitor travel time, Medidata Remote Source Review expedites time to review documents and data improving clinical trial management efficiency.

Ensure Clinical Trial Data Quality & Compliance
Medidata Remote Source Review is a 21 CFR Part 11-compliant system and protects PII and PHI with built-in redaction functionality that helps reduce errors. Intelligent workflows and flexible permissions enable automatic distribution of your source documents to the right monitors for their assigned sites. Automated source document removal, once SDR/SDV is complete, ensures compliance with regulatory requirements. A full audit log and documentation helps you track and re-verify data, reducing the risk of failing an audit.

Gain Clinical Trial Oversight, Control, and Visibility
Remote Source Review provides a series of standard reports for task management and status updates and improves your oversight with a full audit trail that captures all activities. Electronic documents are available for your review immediately upon upload.

Clinical Study Support
Implement with confidence. Medidata’s Professional Services team of experts has countless years of clinical trial experience and a deep understanding of the industry. We partner with you every step of the way to ensure your remote SDV/SDR processes are optimized right from the start.
Learn more about the partnership between Professional Services and this leading global medical device company’s implementation of Remote Source Review.
Key Features of Remote Source Review
Submission & Review
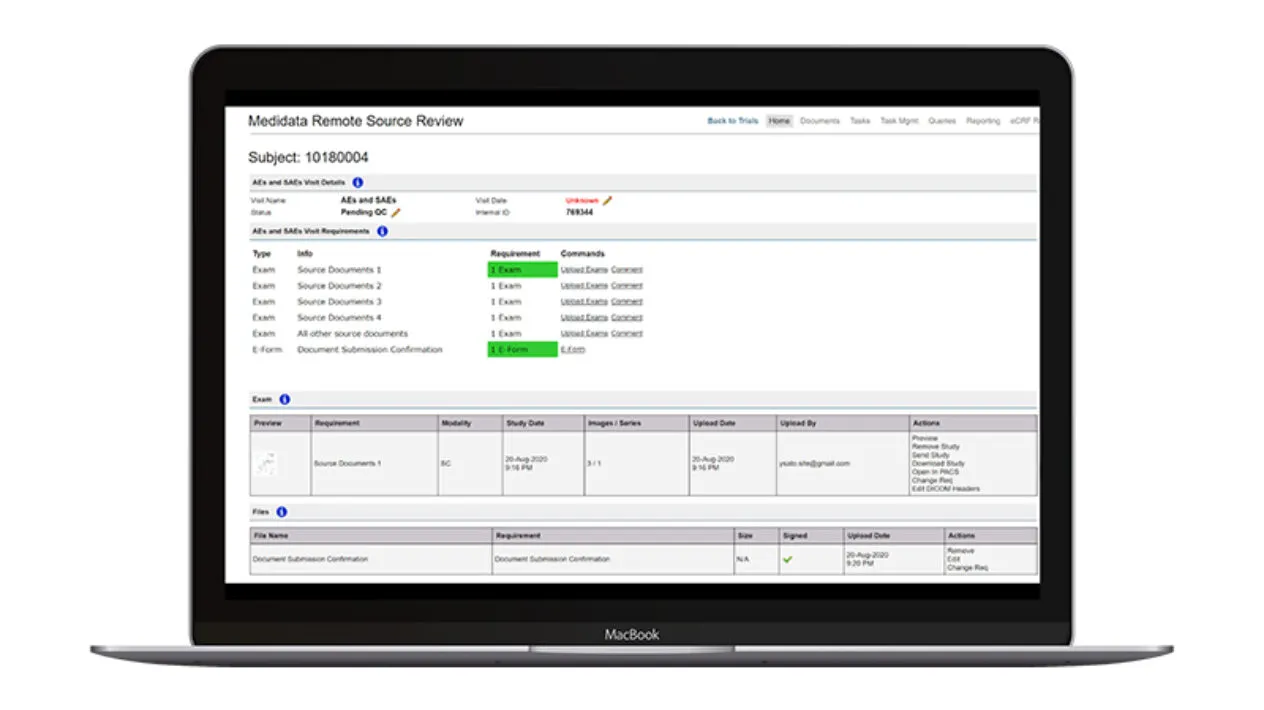
Remote Source Review provides a structured submission process to minimize errors with a prespecified workflow to support critical document management and SDR activities including mobile device upload options.
Sites have minimal manual data entry with automated patient entry when connected to Rave EDC. Sites and monitors have a consistent and seamless user interface and preconfigured query text to aid in site/monitor communication. Automated source document removal once SDR/SDV is complete ensures compliance with regulatory requirements.

Workflow Management
Remote Source Review offers intelligent task management and document routing with conditional logic to ensure all tasks are in the right place for the right site. Standard reports for task management and status updates are available so monitors know the status of the visit.

Security & Compliance
Remote Source Review includes many features that enhance the security and compliance of your data:
- Search and find feature for automatic redaction
- Tracking of tasks with completed forms
- 21 CFR Part 11-compliant system
- Audit ready—accelerated resolution of audit findings
- Process for systematic permanent document removal
Related Solutions
Learn More

Case Study
Learn how Frontier Science keeps HIV/AIDS Clinical Trials moving with Remote Source Review in partnership with Medidata Professional Services.
Remote Monitoring with Remote Source Review
Secure, remote monitoring of critical source documents helps keep your trials running smoothly. Rapidly and remotely enable monitors in critical document acquisition, workflows, and both Source Document Review and Source Data Verification, to more quickly assess patient safety and data quality.

Modernizing Clinical Trial Oversight
The rising complexity of clinical trials, combined with pressures resulting from the COVID-19 pandemic, have forced sites, sponsors, and clinical research organizations (CROs) to adopt remote and risk-based approaches for clinical trial execution to ensure the safety of trial patients, maintain compliance with good clinical practice, and minimize risks to trial integrity.

CTI Adopts Remote Source Review
Medidata partners with CTI for Remote Source Review of Clinical Trials to power remote monitoring and document review for multiple global studies, including several COVID-19 projects, and provide a scalable solution to take them beyond the pandemic.

Drive Efficiencies and Speed for Your Site with Secure, Remote Monitoring
Learn more about how Medidata Remote Source Review allows your site to easily manage all monitoring-related tasks. Drive efficiency and speed with secure remote document review, based on easy-to-use technology that ensures data quality and compliance.