ICYMI Vol 2 Issue 1

Happy New Year! With a new year comes new challenges. Find out how Medidata can help you Solve the Impossible together by requesting a platform demo. Click here to sign up.
TIP OF THE MONTH
Q: In Rave EDC, how do I remove a Principal Investigator (PI) signature from a form / forms without needing a script or having to make the PI change data?
A: If the volume of forms requiring re-sign is relatively low, you may opt to inactivate; then reactivate the eCRF(s) through subject administration in EDC. This will break the signature, meaning that sites are required to sign the whole form(s) again.
PRODUCT UPDATES
FTP Access to Medidata Production Servers
Important Action Required!
As of January 28, 2023, the settings for accessing FTP clients will need to be updated. Otherwise, you will not be able to access the FTP to drop or pick up files.
Simply update "Port" and select the appropriate Connection in the dropdown menu, as shown in the sample below:
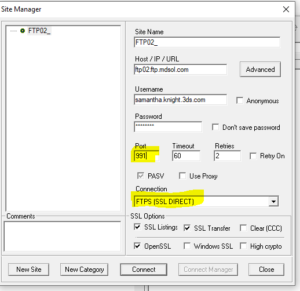
For more details, check out How to Configure Selected FTP Clients and Customer Guide - Secure FTP Transition.
Data Reviewer
Build Complex, Multi-Dataset Listings without Programming
In H2 2022, Medidata Detect’s Patient Data Surveillance (PDS) module was enhanced with a new Data Reviewer capability. Data Reviewer presents datasets aggregated from Rave EDC and other sources and enables data managers to build complex listings, joining data from multiple domains, using a drag-and-drop expression builder. Listings can be used to find exceptions and, for data originating from Rave EDC, automatically post queries in bulk to the appropriate patients, visits, forms, and fields in Rave EDC, saving a lot of time otherwise spent manually posting them individually. In 2023, the capabilities will be expanded with AI-driven data reconciliation for adverse events, concomitant medications, and medical history.
Learn more about Medidata Detect’s PDS and Data Reviewer capabilities:
- Patient Data Surveillance Factsheet
- Data Reviewer Demo Video (under 3 minutes)
Medidata User Experience -- NEW!
In the spirit of continuous improvement, we will be enhancing our homepage, navigation flow, and notifications for a more user-friendly experience. Customers will be able to access information on the Medidata platform easily through the new shared design, language, and overall experience. This step of the evolving Medidata platform will include a newly designed homepage as well as an enhanced global navigation process.
On the homepage, users will soon be able to easily access notification center and a central page where users can securely read study-related alerts and events. This will be rolled out in phases to segmented groups; please send an email to medidata.partners@3ds.com for more info.
COMMERCIAL DESK
We've made a few changes to the Bid Request form regarding Medidata Detect. It now includes fit-for-purpose modules developed to fit specific user activities and analyses, as follows:
- Patient Data Surveillance (PDS) module -- now includes Patient Profiles and Data Reviewer. It also provides in-depth, subject-level data to ensure medical congruence between all data sources
- Patient Data Surveillance (PDT) + KRIs modules -- consolidated, user-friendly view into sites' currency and performance utilizing data from various sources
- End-to-End Data and risk surveillance -- available across the trial, breaking silos between operational users
Read more here.
TRAINING & CERTIFICATION
Global Education
The Global Education Certification and Training subsites have been refreshed to enable access to global education content updates, new certification programs and updated role-centric Learning Paths (now available upon request). Send an email to medidata.globaleducation@3ds.com for more info.
PARTNER PROGRAM
PartnerConnect
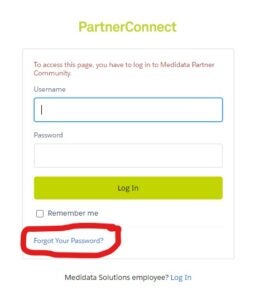
Here's a best practice ... use the "Forget Password" option. Make sure you are using the correct email convention; it should be account name@3ds.com.mdpc. See below for a sample:
Besides submitting bid requests, did you know you can download marketing collateral, such as fact sheets, videos, and other product-related content under the Resource Hub in Medidata's Partner Community portal? If you need a refresher on how to navigate PartnerConnect, send an email to medidata.partners@3ds.com to request training.
AWARDS & RECOGNITION
Welcome to Medidata Partner Program
Welcome Pacesetter! We look forward to a long and successful partnership.
Partner Anniversaries
Thank you Cognizant for 10 years and Westat for 5 years of partnership!
Click on banner to view message from Westat
Partner Accreditations
Congratulations to these partners for achieving new accreditations!
- IQVIA -- Site Cloud
- Medpace -- Site Cloud
- Clinfocus -- Rave EDC
NEWS & EVENTS
Events
NEXT Boston -- April 25 (Save the Date!)
Awards
Medidata Link
- The first centralized solution that connects patient-level clinical trial data and real world data, receives the PM360 Innovative Product award. Read more

- Medidata receives prestigious innovation award, with Medidata Link being honored for bridging the gap between clinical trial and real world data, advancing regulatory science and improving public health. Read more

WHAT TO LOOK FOR IN THE NEXT EDITION
- DCT Sales Enablement Update
- Changes to Your myMedidata Experience
- Learn more about where to find Product Enhancements & Updates -- watch next issue for video tour
Finally, we welcome comments and suggestions for future topics. Our goal is Connecting with Partners to Solve the Impossible. We will continue to find new ways to grow and cultivate a leading partner program for you! Please contact us via email medidata.partners@3ds.com
