Medidata eTMF
Faster Document Management with a Simple, Automated Trial Master File Solution
Medidata eTMF (Electronic Trial Master File), part of the Medidata Study Experience, streamlines and automates the creation, management, and oversight of clinical trial documents, ensuring the TMF is always complete, and inspection-ready.
Medidata eTMF Benefits
Streamlined Study Conduct
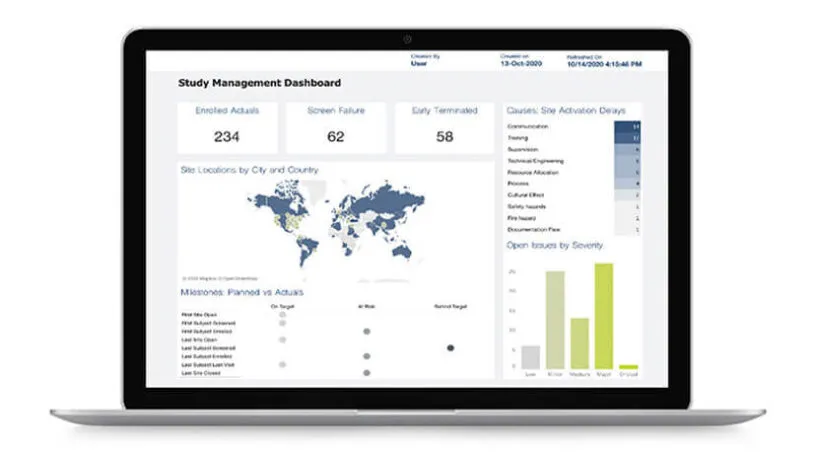
Medidata eTMF, unified with Rave EDC and Medidata CTMS on the Medidata Platform, provides a single end-to-end solution for managing your study and document data. The result is a unified solution that provides a complete picture of your clinical trials’ progress, ensures you are inspection ready, and frees up time and resources so that you can focus on the most important tasks.
Faster Path to Success
Medidata eTMF is delivered using proven, agile planning and implementation methodologies. Typically, Medidata eTMF installation takes only eight weeks from kick-off to go-live.
Simplify TMF Management
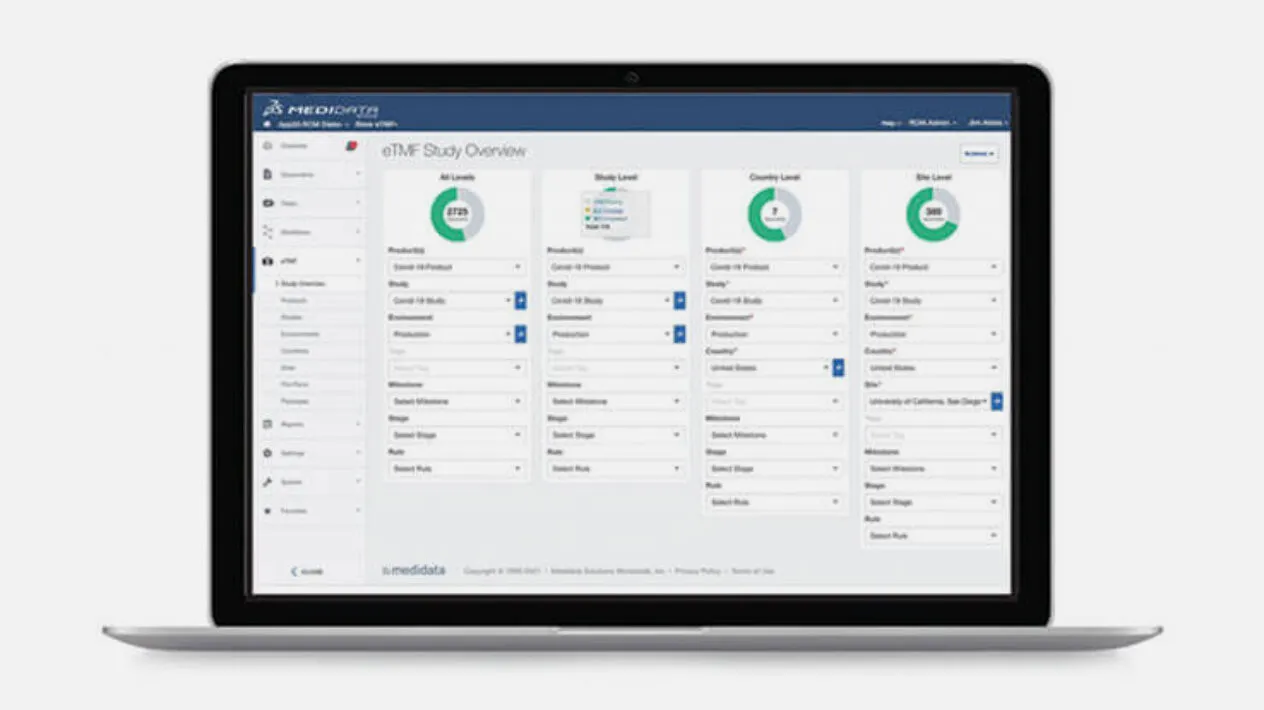
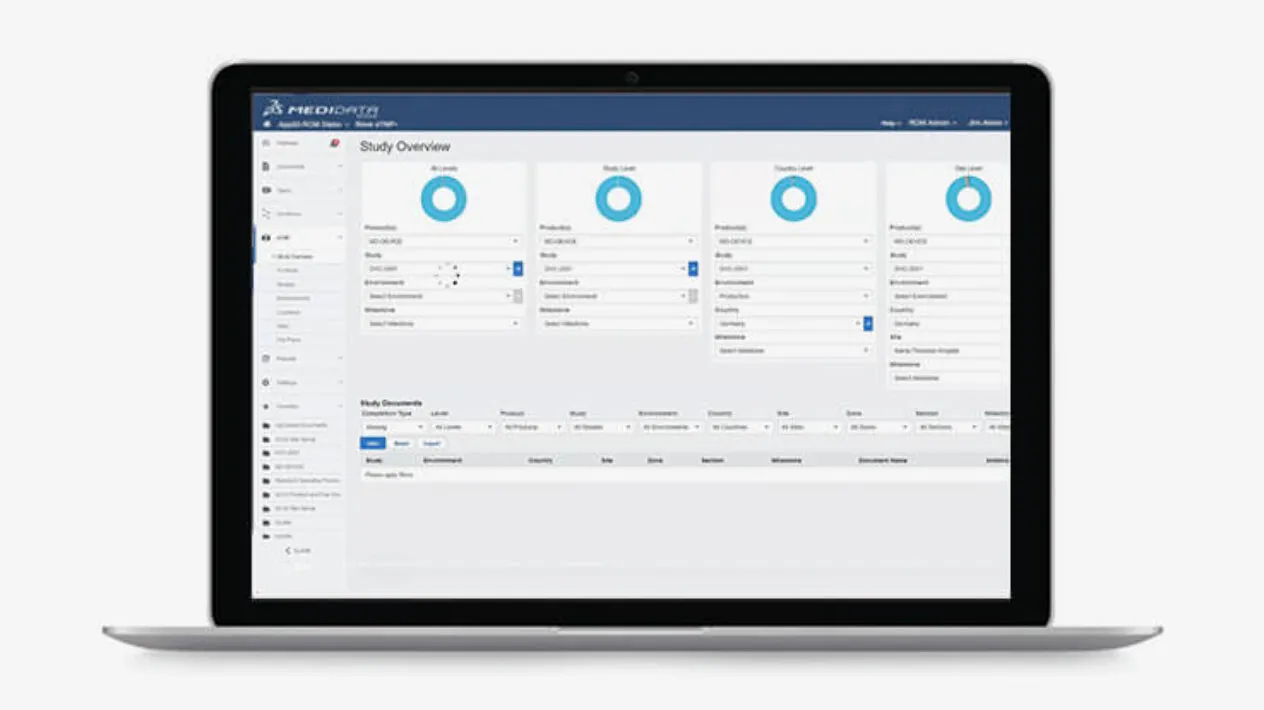
Medidata eTMF simplifies the clinical document filing process by combining content and data from the study’s entire life cycle. Because it is part of the Medidata Platform, Medidata eTMF can instant auto-populate content and data from other applications on the platform, so your TMF is always complete. New study plans are generated in minutes and file plans can be customized.

Strengthen Real-Time Collaboration
Whether you’re a site, sponsor, or CRO, you can create, store, view, edit, and jointly work on an entire TMF life cycle in a single, intuitive application.
Key Features of Medidata eTMF

Comprehensive Search
Medidata eTMF’s advanced search algorithms, based on content, title, and/or metadata, make searching your TMF artifacts simple and accurate. Powered by auto-naming and metadata, Medidata eTMF provides standardized content, so you can easily search and manage both regulated and non-regulated content in a single platform.

TMF Reference Model Included
Medidata eTMF provides you with full support for the Drug Information Association’s (DIA) TMF reference model, and includes an out-of-the-box DIA file plan configuration.
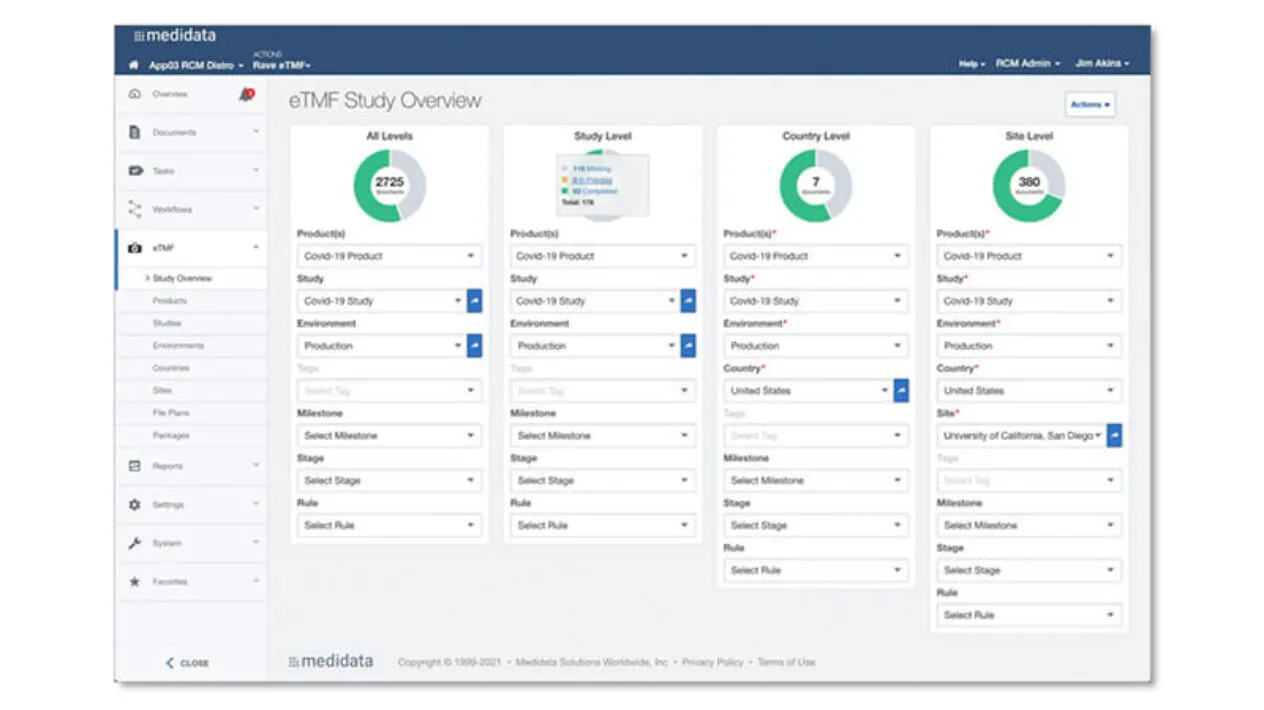
Dashboards and Reporting
Medidata eTMF provides real-time oversight and access to your documents through comprehensive dashboards and reporting, ensuring you maintain a constant state of inspection-readiness.

Intelligent Placeholders
Medidata eTMF provides you with an intelligent document placeholder tool, which creates document package placeholders based on events that occur over the course of the trial. You can also create and trigger packages on demand.
Related Solutions
Learn More

Simplify Trial Oversight with Unified Document Management
Increasing TMF complexity requires the management and strict regulatory filing requirements of often thousands of documents per day. Increase operational efficiency with enhanced collaboration, real-time oversight, and automated document workflows with Medidata eTMF.

Automated Clinical Trial Monitoring Workflows Make a Lean Team More Efficient
When Enterin needed a solution to automate the generation of confirmation letters and reports and also make it easier to share that data with senior leadership and site managers, they turned to Medidata. Adopting Medidata eTMF, Enterin realized a savings in time of 5-6 hours per week and decreased site burden.