Medidata RTSM
Bringing Innovation and Convenience to IRT
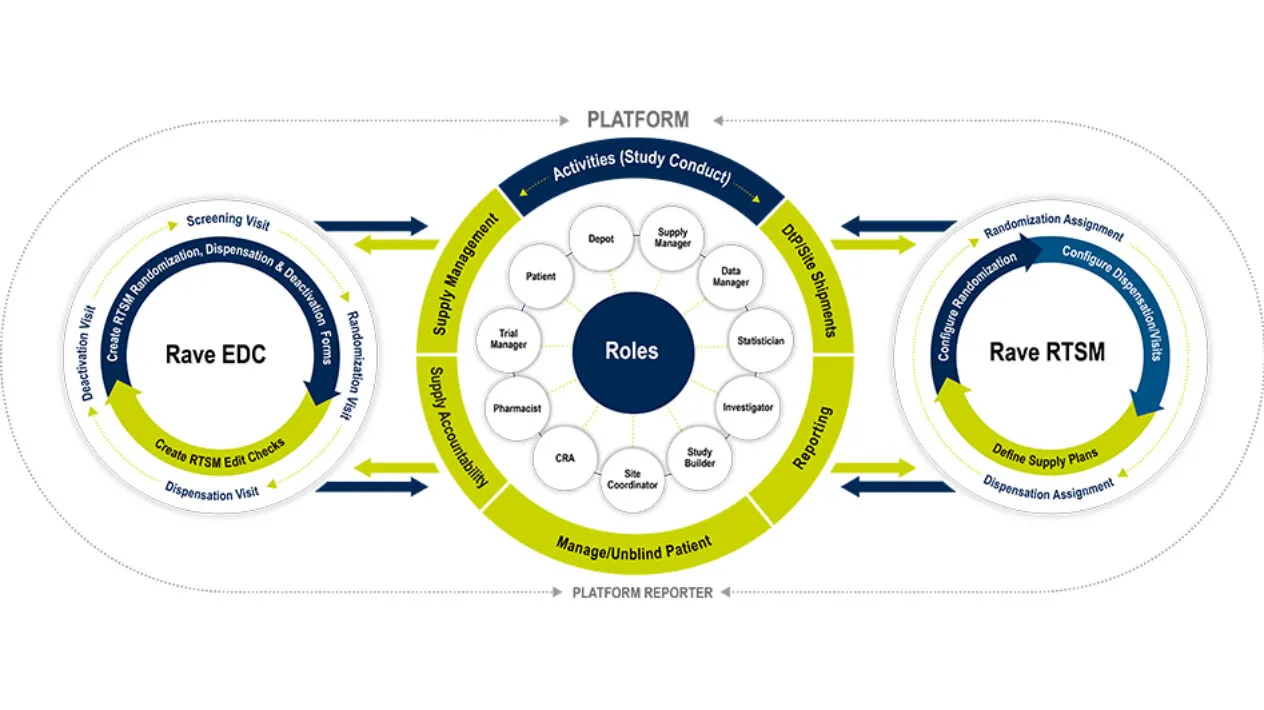
Medidata RTSM is a robust patient randomization and trial supply management system that serves as a centralized control center for patient randomization and drug logistics. It is designed for simple to the most complex clinical trials. With RTSM on the unified Medidata Platform, you have one central hub for your data and user experience. Like all Medidata products, it’s designed with the end-user in mind and allows Sponsors and Sites to meet First Patient In (FPI) quickly, to then handle mid-study changes with ease. Visibility is no challenge with RTSM’s powerful reporting and dashboards that can significantly reduce reconciliation time. Medidata RTSM has proven to enable you to meet your study milestones sooner.
Medidata RTSM Proven Track Record
Right Patient, Right Drug, Right Dose, Right Site, and Right Timing
Studies
Studies across various therapeutic areas
Patients
Patients per month across complex studies
Countries
Countries supported
Years
As a trusted IRT Partner
Years
Services team’s average clinical trial experience
What Customers Have to Say
“After 30 years in Pharmaceutical Supply Chain, I have found Medidata Rave RTSM to be the most complete and user-friendly IRT system to support clinical supplies.”
– Todd Zegarzewski, Sr. Supply Chain Manager
“Rave RTSM and the RTSM PS team complemented each other perfectly in our study. Especially in complex studies with different study materials, Rave RTSM is extremely helpful in maintaining an overview at all times. We are able to control the flow of study materials and make adjustments to the inventory on our own in Rave RTSM. Medidata’s teams were always ready to help with any questions and could always rely on the competent and quick help of our contact persons. All in all, we were absolutely satisfied with the tools and services offered!”
– Dr Bernd Ullrich, Senior Clinical Trial Manager, Heidelberg ImmunoTherapeutics
“Medidata has made a tremendous impact on how MyMD Pharmaceuticals has been able to conduct its clinical research. Their comprehensive suite of services has simplified aspects of the clinical trial process, from randomization [with RTSM] to data collection and analysis. Through the platform’s intuitive interface, our research teams were able to confidently navigate the complexities of the trial to ensure all data elements were captured efficiently. Medidata has also helped reduce the administrative burden of data collection allowing the research teams to focus on patient outcomes. I look forward to working with Medidata [Professional Services] on future trials..”
– Jenna Brager PhD, RN, Executive VP, Drug Development, MyMD Pharmaceuticals, Inc.
“The Medidata RTSM Professional Services team has been outstanding in their efforts to support our business needs. Their Live Study Management (LSM) service has been invaluable, as we have a dedicated RTSM SME resource who can guide us in using RTSM throughout the entire live study phase and can address our RTSM questions. They will also make data changes in RTSM on our behalf as an unblinded resource. Their expertise and quality of service have been extremely beneficial to our study team and instrumental to our study’s success.”
Benefits of Rave RTSM
Key Capabilities
Robust Cohort Management
Enables seamless mid-study changes and new cohort additions for real-time cohort control for adaptive trials.
Edit Live Design (ELD)
Apply protocol updates after go-live with no downtime to reduce delays and stay on track.
Visit Cycles
Configure repeating visit series over fixed or flexible timeframes to simplify setup and reduce errors.
Multi-Dose Vial
Track vial use across patients and monitor remaining dose for accurate dosing and inventory management.
Electronic Supply Accountability (eSA)
Eliminate paper logs with Rave EDC-integrated eSA to streamline closeout and improve IP data accuracy.
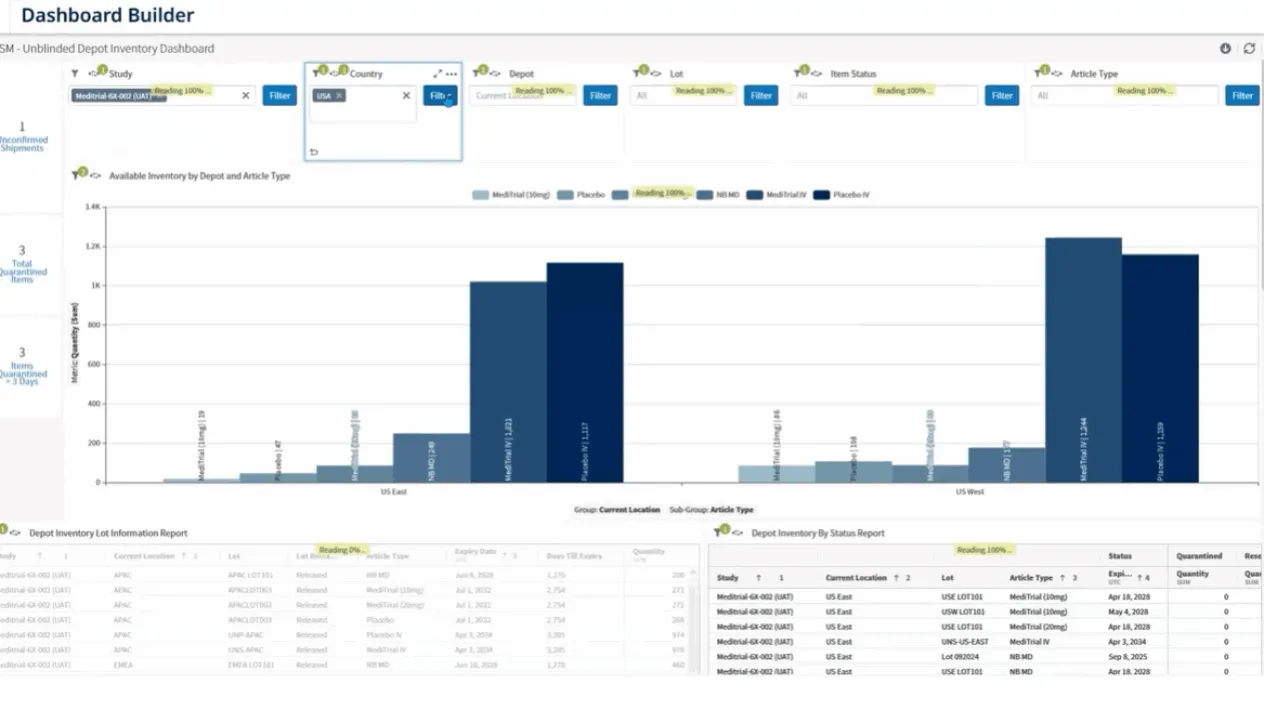
RTSM Analytics
Access real-time dashboards and custom reports for oversight of randomization, inventory, and shipments.

Direct-to-Patient (DtP)
Trigger DtP shipments at site, visit, or patient level. Patients confirm receipt via myMedidata to support decentralized models.
Depot Integration Service
Reduce manual coordination and delays with pre-validated two-way depot integration for direct site and patient shipments.
Supply Management
Adapt inventory in real time using predictive logic to match demand and reduce overages.
Forecasting
Plan supply needs to prevent stockouts and optimize labeling and supply.
Drug Pooling
Maximize efficiency by sharing inventory across studies, sites, and depots with pooled supply strategies.