Preparing for a New Data Future: Perceptions of eClinical Architecture
This blog is part three of a five-part series on the findings of this survey of clinical research technology decision makers. Download Preparing for a New Data Future: A Survey of Clinical Research Technology Decision Makers for comprehensive survey results to guide your unified platform approach to clinical trials.
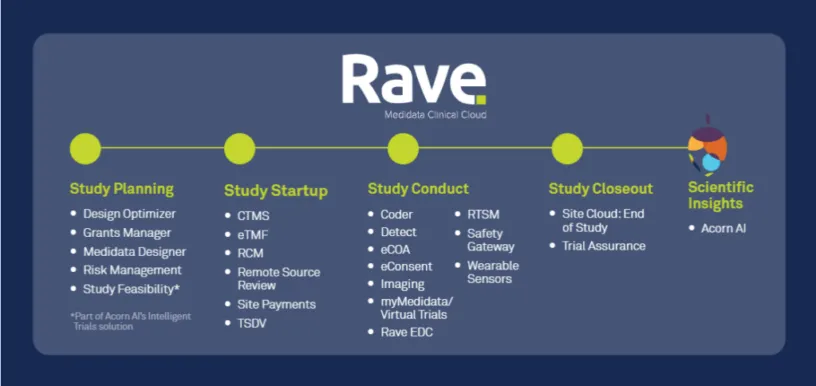
A rapid shift towards virtualization due to the COVID-19 pandemic, larger volumes of data collection from an increasing number and diverse set of data sources, and the need for sponsors and CROs to gain more complete pictures of trials and to act on data quickly are forcing the industry towards eClinical software solutions that are on unified platforms. Our new survey of key decision makers in clinical research technology examined industry outlook on eClinical architecture. Key findings include:
The biggest barrier is resistance to change
Life science tech leaders expect a slow convergence to a unified platform architecture across the industry. However, it remains inherently difficult for organizations to make a platform change as they operate ongoing studies.
Organizations see the benefits
Respondents cited several business-case benefits for using platform systems, including lower total cost of ownership, increased study speed, easier access to relevant data and reporting capabilities, and fewer manual processes.
The best organizations take advantage of change
Organizations that have shifted to an external eClinical solution provider-based platform have sought opportunities to make the case for a change (e.g., contract renewal, step-change in studies, business event/transaction).
eClinical software solutions architecture will take on different forms
eClinical software solutions architecture for pharmaceutical/biotech companies is likely to come in one of two forms:
- Large legacy companies: Platform architectures are often entirely or mostly built internally, with a mix of cloud-hosted solutions and internal integration. These complex systems carry high costs, and deployments of new applications often require reconsideration of the existing architecture.
- New companies: A precipitating event (such as M&A or a new venture) often leads to considering a new or modified architecture. Few businesses in this situation will invest in internal IT development; they consider either outsourcing their studies to CROs or procuring an eClinical platform that covers all or most of their needs. SaaS solutions that offer end-to-end coverage and easy integration to other needed capabilities are critical factors.
Similarly, eClinical software solutions architecture for CROs is also likely to come in one of two forms:
- Preferred tech: These are typically large CROs that go to market selling their outsourced services in addition to their own technologies. These technologies may be the result of internal development, acquisition, or exclusive vendor partnerships. These CROs may prefer to rely on their own technologies, but they also use and sell others.
- Agnostic: These CROs have not built large internal infrastructures, but they can typically manage data integrations. These companies may have a preferred eClinical solutions provider they recommend but will typically follow the client’s preferences.
The pharmaceutical industry will continue to evolve and adapt to its increasingly digital environment and move from single and fragmented point solutions to unified platforms. Embracing unified platforms eases the burden on clinical development teams, scale to support large numbers of studies, and allow for sponsors to centralize their data to gain powerful insights across trials instead of dealing with disparate datasets isolated across several point solutions.
Stay tuned for the next edition of this five-part series. Download Preparing for a New Data Future: A Survey of Clinical Research Technology Decision Makers for comprehensive survey results to guide your unified platform approach to clinical trials.
Contact Us
