The Medidata Platform: One Place for Your Clinical Trial Needs
The Medidata Platform connects patients, sites, sponsors, and CROs in a unified, secure, and scalable cloud environment to bring life-changing treatments to those who need them most.
- Use a common user experience across all your trial tasks
- Centrally manage your clinical and operational data
- Easily integrate with your existing systems
- Accelerate your workflows with automation and AI
Proven to Transform Your Clinical Trial
It’s not just talk. Medidata customers are seeing real impact on their studies when using our solutions and services, reaching their study milestones in record time.
Reduce Your Study Builds by
ONE MONTH
When using Medidata Professional Services
Conduct Studies
FIVE MONTHS
Faster when using Multiple Products
Reach Database Lock
FOUR DAYS
Sooner when using Multiple Products
- Analysis of difference in median build time vs. matched studies not using PS (p<0.05); Reduction of 30 days.
- Analysis of difference in median FPI to LPLV time for EDC + at least one additional product vs. EDC only studies (p<0.05) 2017 to 2021; Reduction of 59 days.
- Analysis of difference in median LPLV to DBL time for EDC + at least one additional product vs. EDC only studies from 2017 to 2021.
Medidata Platform Solutions
As the industry’s only unified platform dedicated to clinical research, we help life science and medical device organizations cut development costs, mitigate risks, and deliver treatments and devices to market faster.
Whether you choose our patient engagement, clinical data management, clinical operations, or trial design and evidence generation solutions, you will have access to the power of the Medidata Platform.
Explore our powerful solutions below.
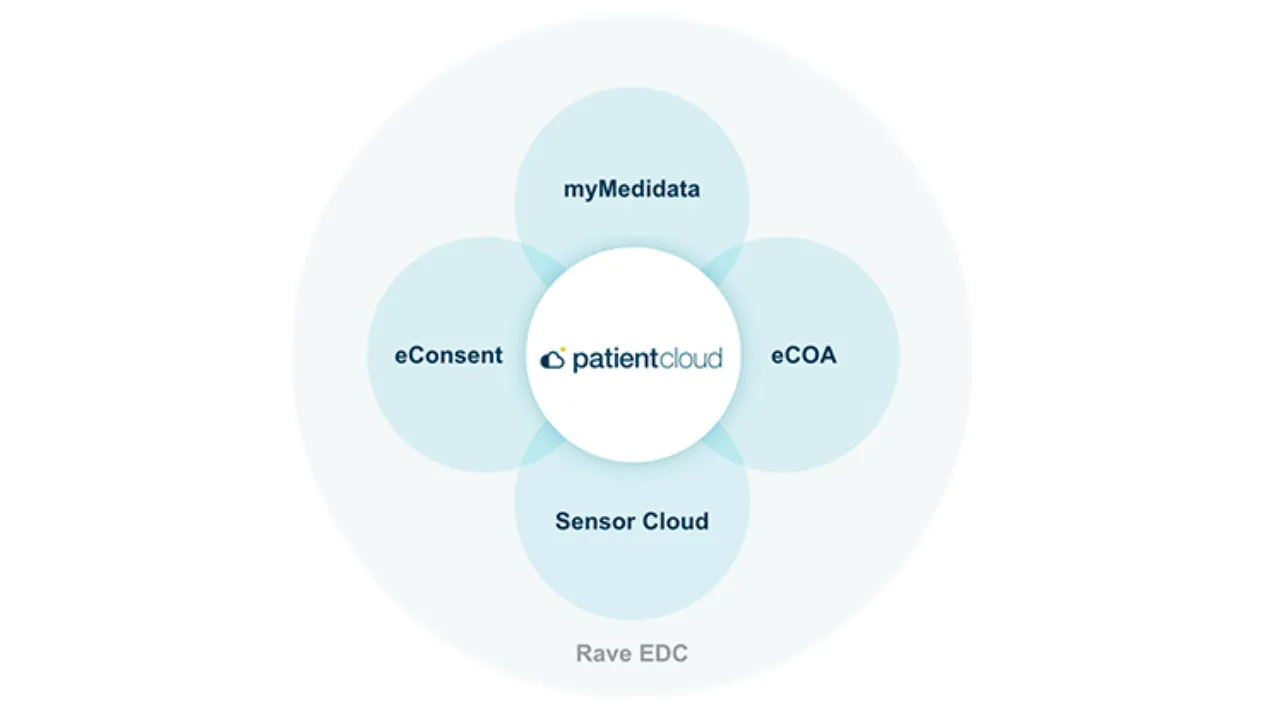
Solutions designed for the patient, by the patient.
Solutions to capture, manage, and ensure the quality of your data.
Solutions to help you better plan and manage your trial.
Solutions that leverage advanced analytics to get your drugs to market, faster.
“So with all the data in Medidata’s one platform, it’s great, it’s easy. …. we’re not downloading from another platform and comparing. It’s all there and it’s easier to integrate.”
– Cathy Hult, Director of Data Management, PROMETRIKA

“So my recommendation with using Medidata, especially …considering the size of my company, is having everything under one hood,…it makes it so much easier when looking at how you’re going to use your resources. You don’t want to have resources spending [an] endless amount of time looking at the interfaces between the various systems you have.”
– Salam Ammus, Executive Director, Clinical Data Management, Alkermes

“I think the consistency across products with Medidata, it’s nice—just the look, the feel. The user experience is similar across the different products which is helpful. And any opportunity to get products to talk to each other is always beneficial.”
– Brian Hunter, VP of Contracts & Trial Management, CNS Healthcare