myMedidata
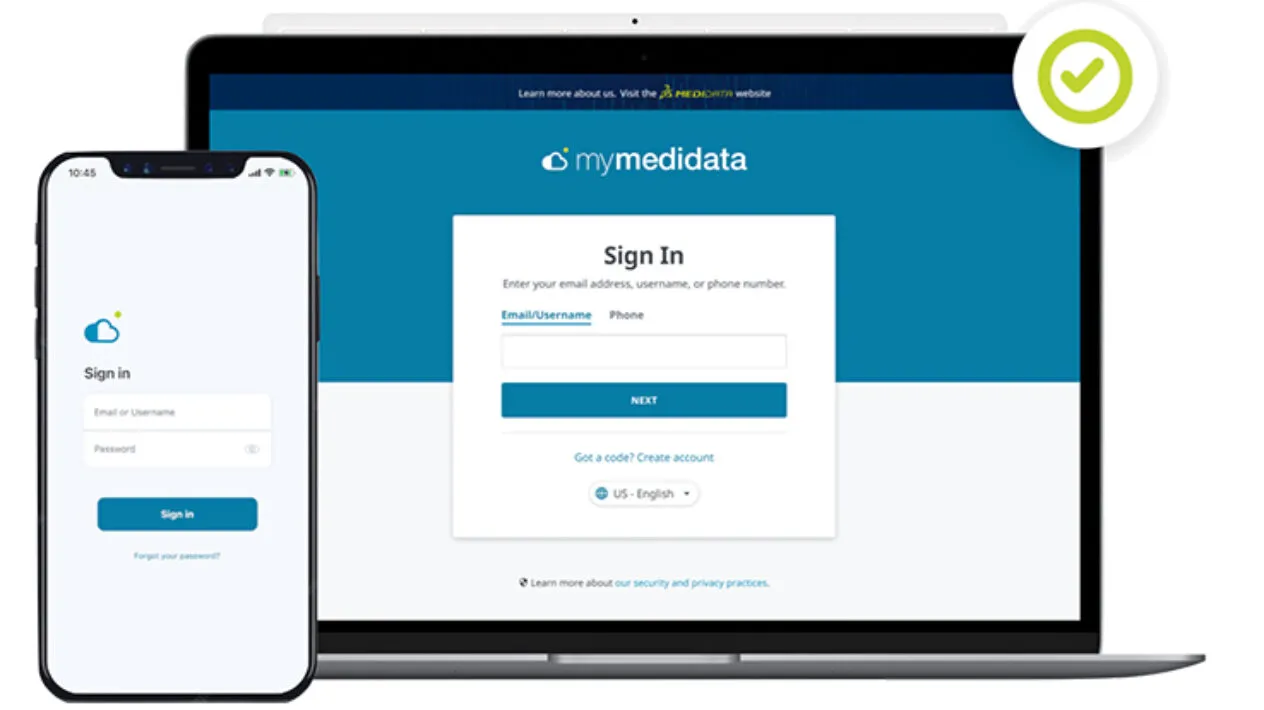
Built for patients, by patients, myMedidata is a single destination patient portal, allowing patients to use any online device to virtually learn, enroll and engage in clinical trials.
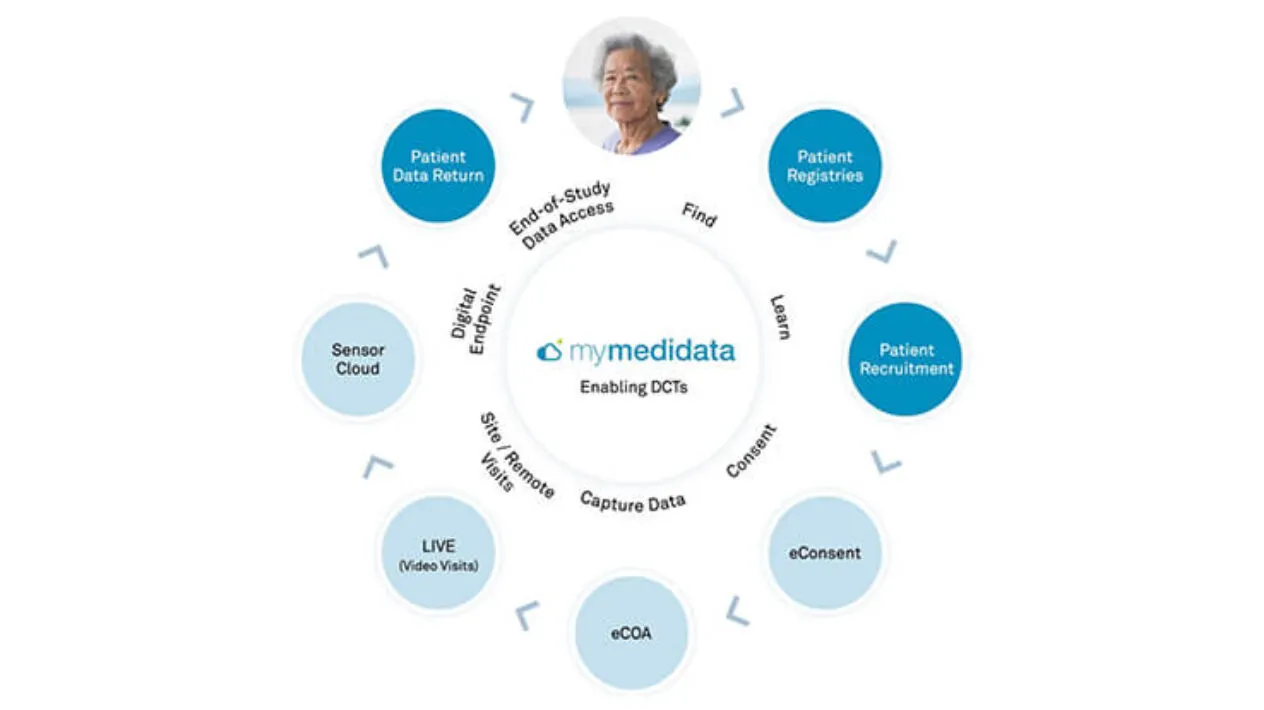
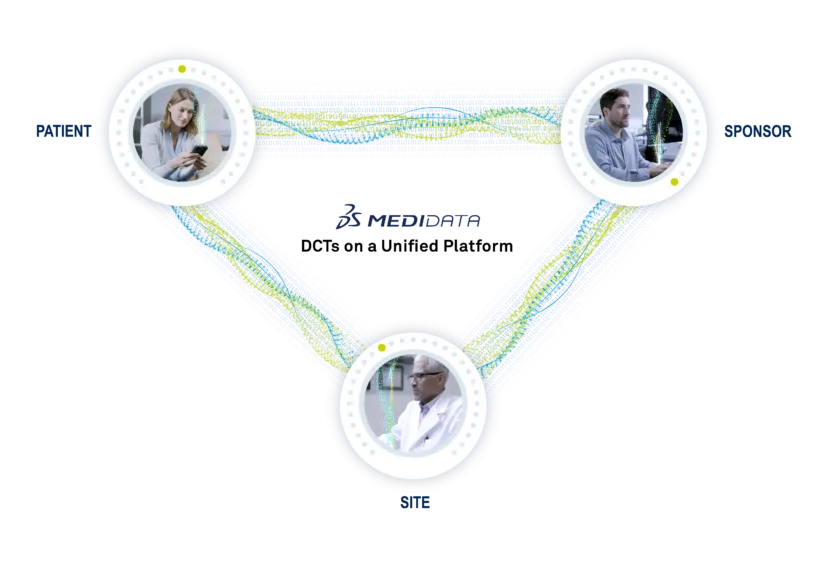
myMedidata, part of the Medidata Patient Experience, enables the full range of tools to build scalable, flexible solutions at every level of decentralized and hybrid clinical trials and our expert teams help you tackle problems creatively to find the most effective level of decentralization.
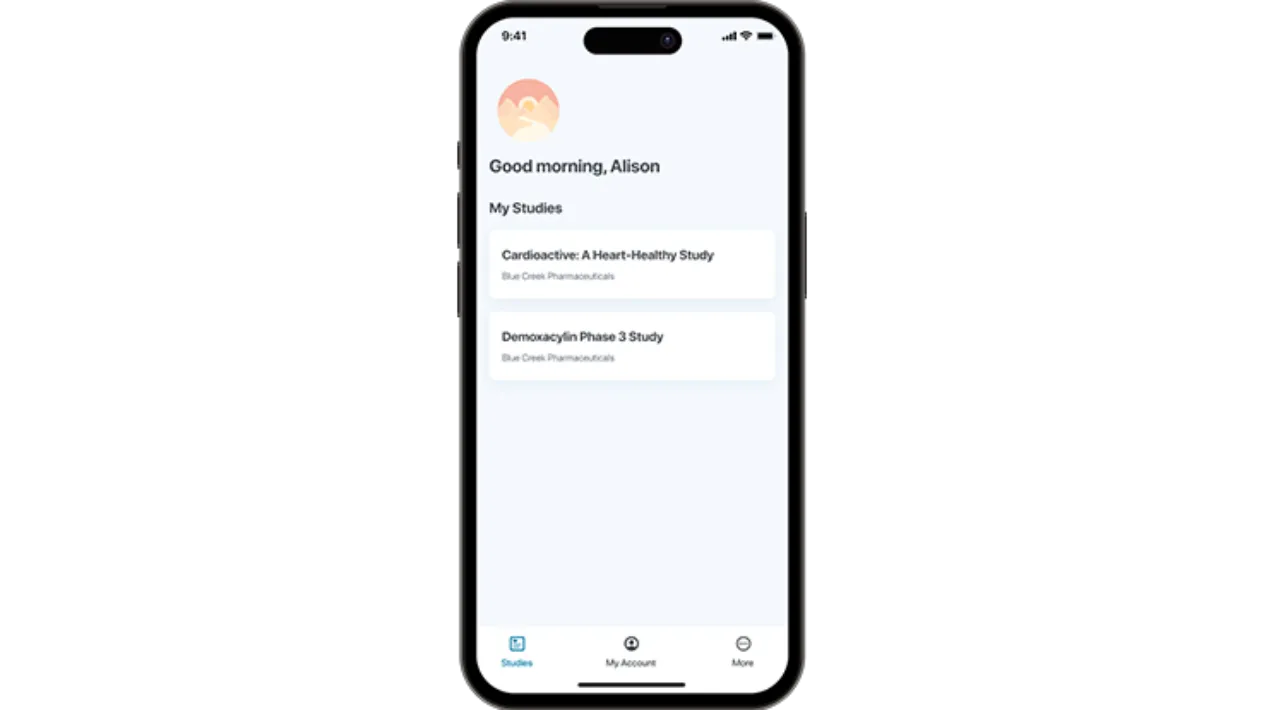
myMedidata App
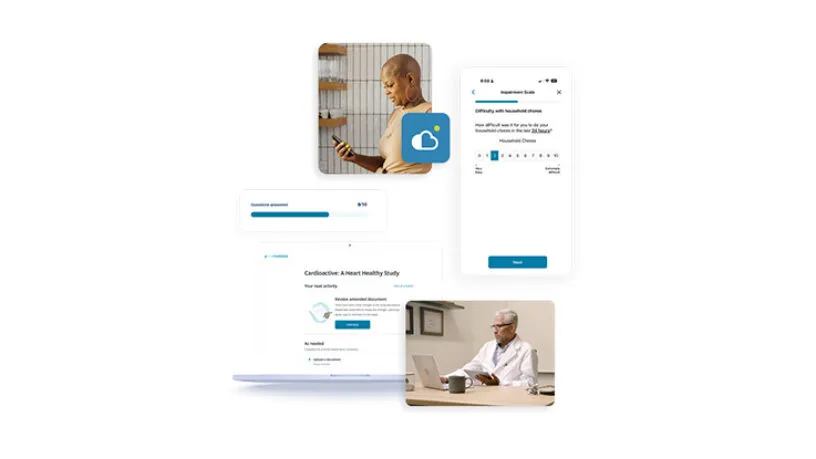
With this powerful native app, patients now have an additional option to easily access their myMedidata patient portal through any mobile device – integrating clinical trial activities seamlessly into daily life.
The myMedidata App is built using Medidata Designer, our new platform configuration tool that builds rich patient experiences through easy to use screen templates, drastically reducing study start-up time.
myMedidata app is available on iOS and Android, and can be used both on the patients’ own devices (BYOD) or via a provisioned device.
Put Patients at the Forefront of Your Studies
Single Clinical Trial Dashboard for Life
Eliminate the need for extra apps, logins, and unnecessary provisioned devices by giving your patients one location for all of their clinical trial activities. myMedidata makes it simple and engaging for patients to participate in any clinical trial so your trials are easier, faster, and produce better results.

Enables Continuous Clinical Data Capture
Empower patients with choice in how they participate in research, so you can efficiently recruit the widest, most inclusive pool of participants, keep them engaged throughout your trial, and produce better study results. Only Medidata allows clinical trials to be more efficient with sites and patients on the same data platform, eliminating integrations and reducing burdens for patients and site staff.
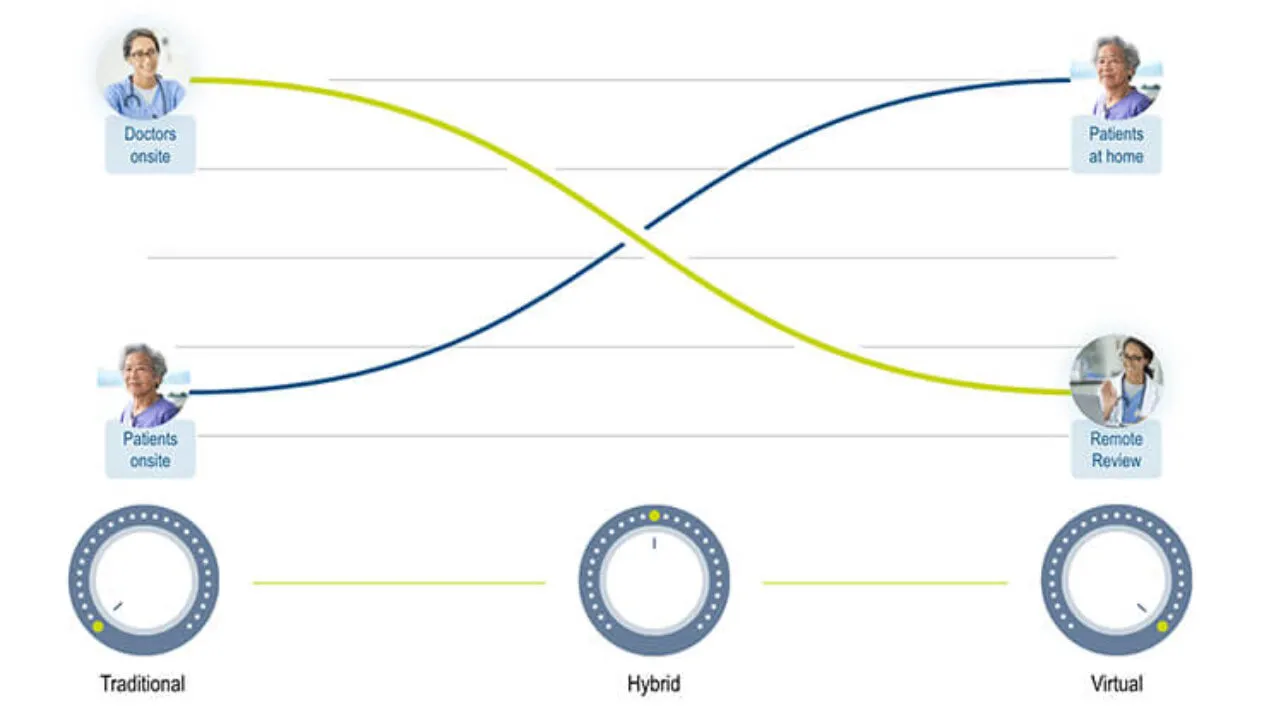
Standardize on Technology
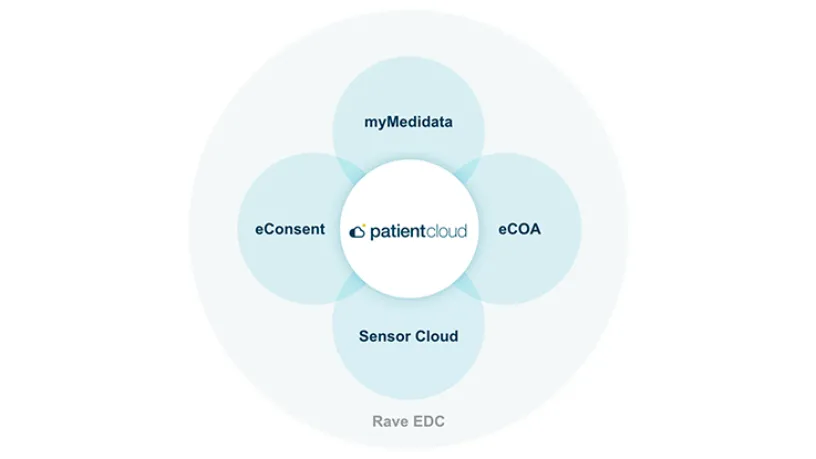
myMedidata is unified with the Medidata Platform – used by the majority of clinical trials worldwide – eliminating the need for multiple workflows, expediting timelines, mitigating risks, and reducing burdens. Only Medidata offers a scalable, end-to-end platform with easy configuration and multiple delivery models based on your specific trial needs.
Key Features of myMedidata

Clearer Consenting Process
To virtually enroll in a new study, patients access their myMedidata account and are then guided through an electronic consenting process known as eConsent. Through myMedidata eConsent, patients watch the study’s eConsent video and review all relevant consent documents. Upon confirming full understanding of the consent, the patient virtually signs their web-based eConsent. myMedidata eConsent can be paired with myMedidata LIVE Video Visits to allow for additional communications and touchpoints between study site staff and patients.
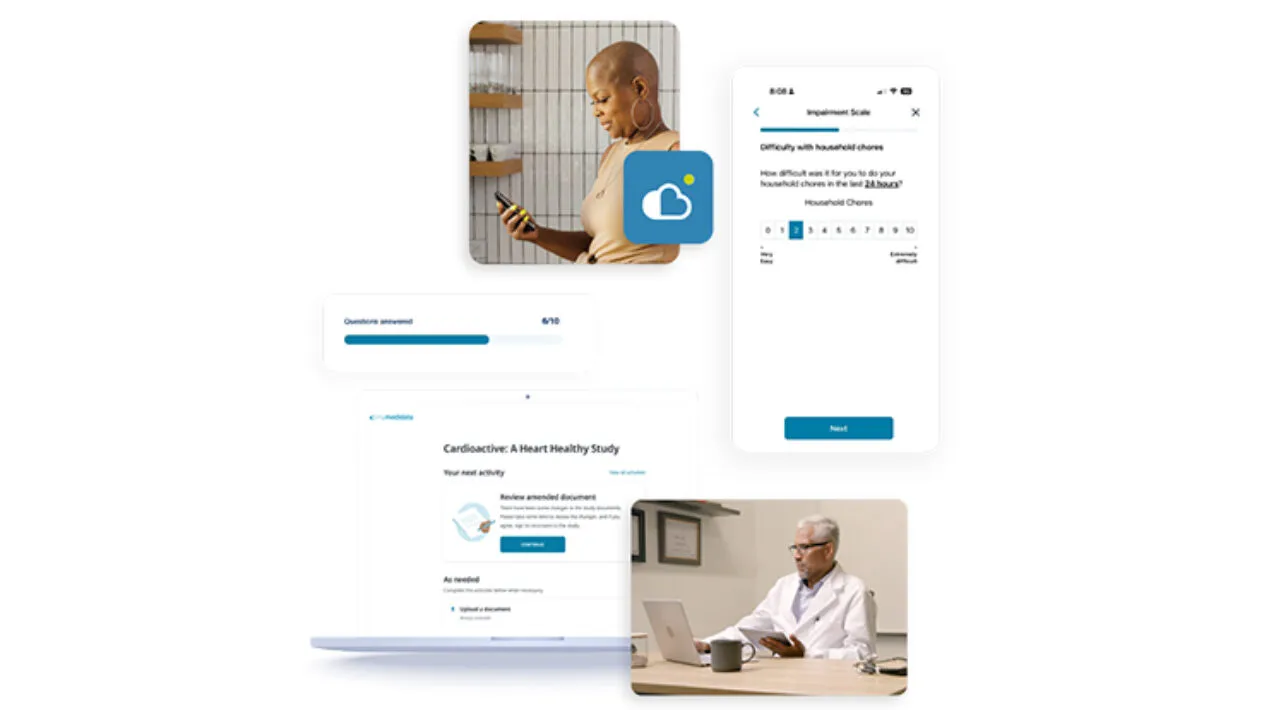
Upgraded Patient Experience
eCOA is built using Designer, enabling Sponsors and CRO partners to build rich patient experiences via intuitive visual workflow tools. Available through the myMedidata native mobile app or any web-enabled device, patients can conveniently access their study tasks where and when it’s best for them. A better overall study experience makes it easier for patients to stay enrolled in trials while expediting timelines and reducing overall costs for sites and sponsors.

Video Visits / Telehealth
myMedidata LIVE is a web-based, live video conferencing capability, virtually connecting patients with their clinical trial study staff. A myMedidata LIVE video visit between patients and sites can replace a scheduled site-based appointment and allow the study teams to complete their data entry in Rave while the patient remains engaged offsite through myMedidata. When used in combination with myMedidata Registries and eConsent, site staff and patients can remain connected without the need for additional travel burdens.
Increased Trial Engagement
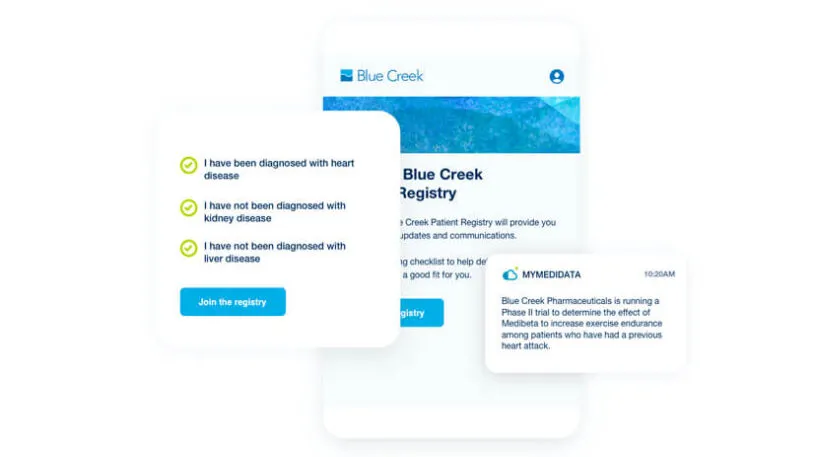
myMedidata Registries expands patient participation from a single trial transaction to pre-and post-trial engagement and patient data return, resulting in a community of educated, empowered, and engaged patients prepared to participate.

Built for Patients by Patients
Medidata’s Patient Insights program infuses the patient perspective into the software development life cycle to create technical solutions that improve the overall patient experience in clinical research operations. Even the most sophisticated innovations in clinical trial technology are meaningless without patient input. To improve patient engagement, Medidata has now expanded access to our Patient Insights Board (PIB) and award-winning Patient Centricity by Design methodology to allow sponsors and CROs to optimize trial design and patient participation.
Related Solutions
Learn More

Decentralized Clinical Trials: The Future of Clinical Research is Here
Download this white paper to read about the changing landscape around decentralized clinical trials, the types of solutions used to run DCTs, and how they benefit Patients, Sponsors, and Sites.

Site Perspectives on DCTs
In collaboration with SCRS, Medidata surveyed sites on their use of DCT solutions and technologies over the last two years. Read this whitepaper to see the results of that survey.