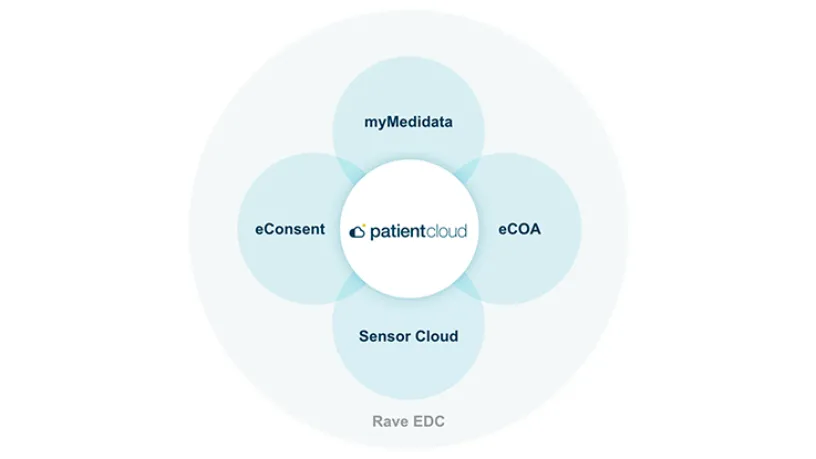
myMedidata Registries
myMedidata Registries, part of the Medidata Patient Experience, expands patient participation from a single clinical trial transaction to pre-and post-trial engagement, resulting in a community of educated, empowered and engaged patients, prepared to participate.
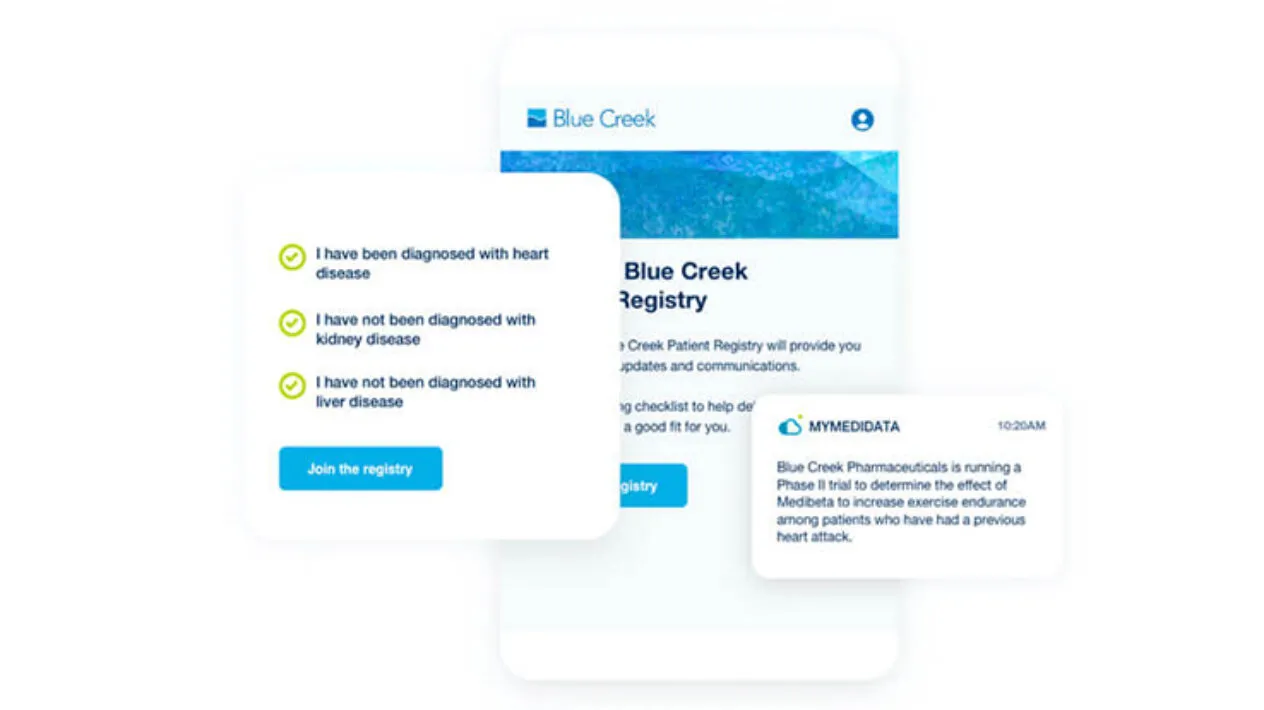
Patient engagement shouldn’t end with a patient’s last visit. With myMedidata Registries, introduce post-trial patient engagement in your clinical trials and enable long-term follow-up, patient data return, enhanced safety monitoring, and gathering of new clinical insights.
myMedidata transforms patient participation from a single transaction focused only on collecting data to lifelong engagement. Patients continue to have access to their portal even after a trial ends, opening up opportunities to learn about other clinical trials.
Transforming Patient Awareness, Access, and Retention in Clinical Trials
Patients
myMedidata Registries drives innovation from the patient perspective because empowering patients with choice is simply the best way to create solutions that optimize recruitment and retention, improve data quality, and shorten trial timelines. By making clinical trial participation more accessible, we aspire to open the door to new therapies, and allow clinical trials to become part of every health care conversation.
Sponsors / CROs
Through effective pre-trial outreach, and post-trial long-term follow up, myMedidata registries educates, empowers, and engages patients beyond a single clinical trial.
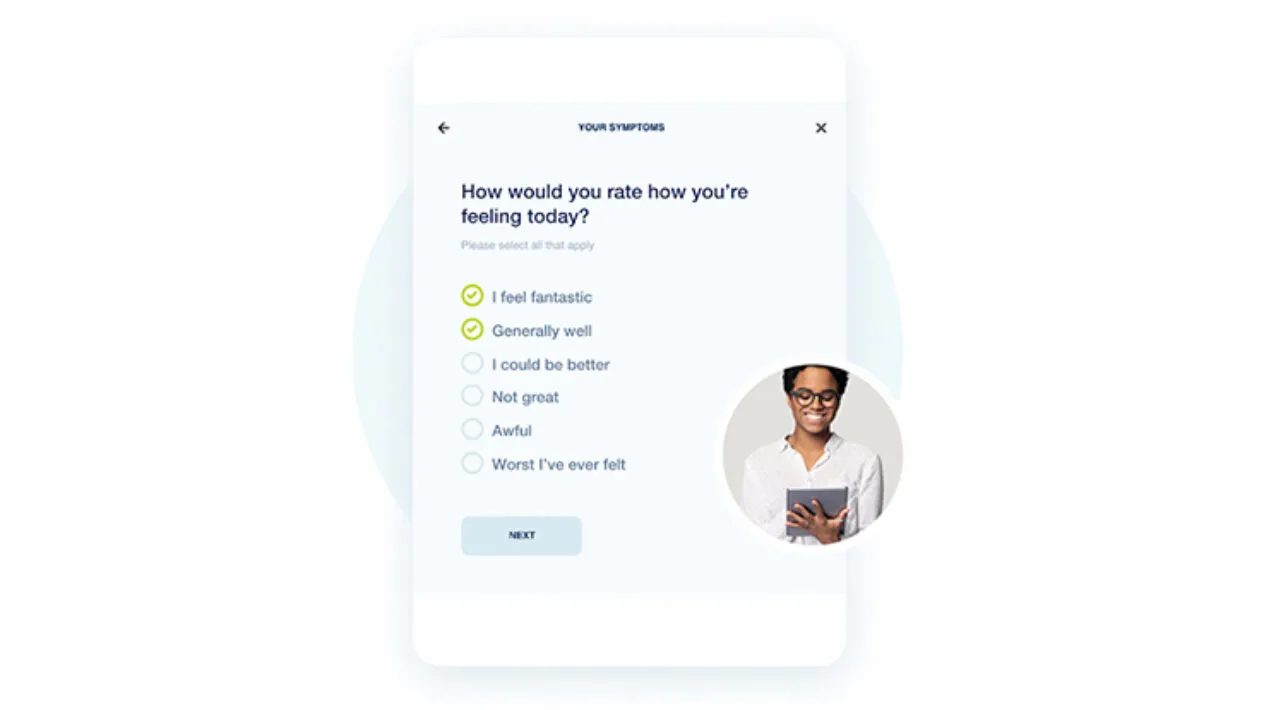
Sites
myMedidata Registries broadens patient access to research, so you can efficiently recruit the widest, most inclusive pool of patients, keep them engaged throughout your trial, and produce better study results. myMedidata Registries make clinical trials faster and more successful by reducing burdens on patients and site staff.
Key Features of myMedidata Registries
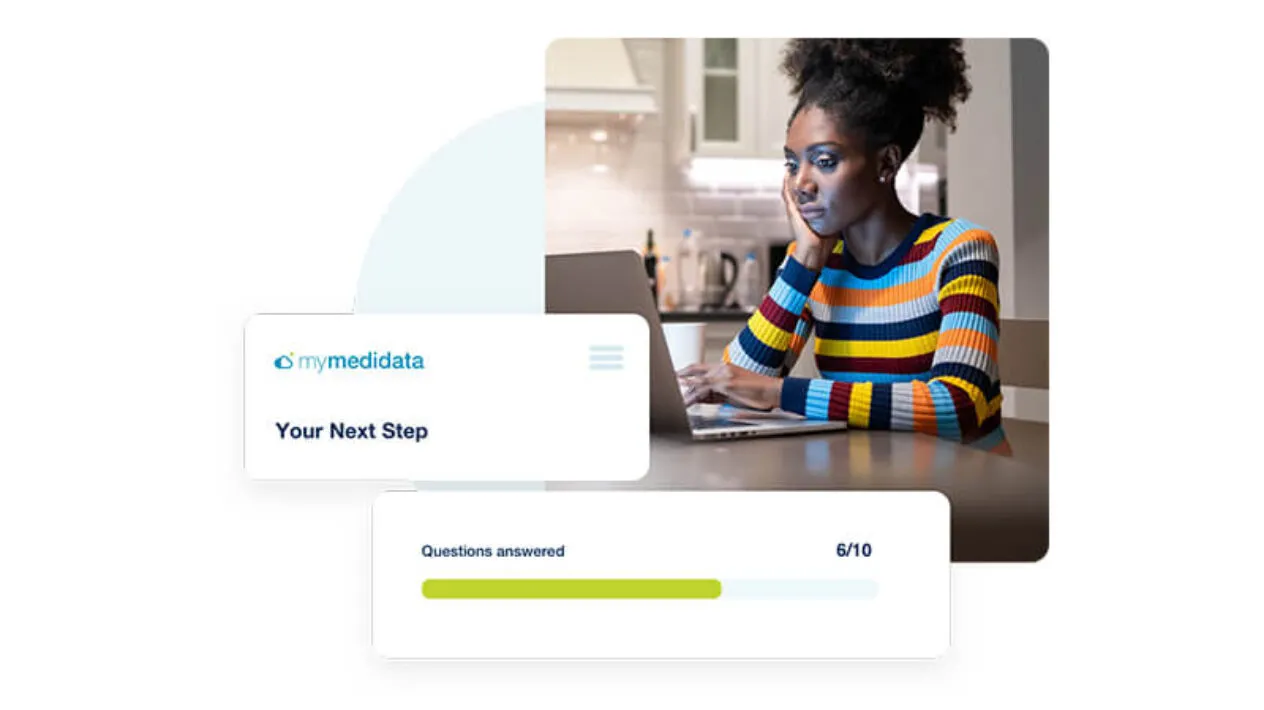
Lifetime Engagement on One Patient Portal
myMedidata Registries provides patients with continuous support in and out of a study with access to one portal, myMedidata, for life. With one login, patients can learn about new trials, be matched with sites, complete forms, and engage in video visits using any web-based device.
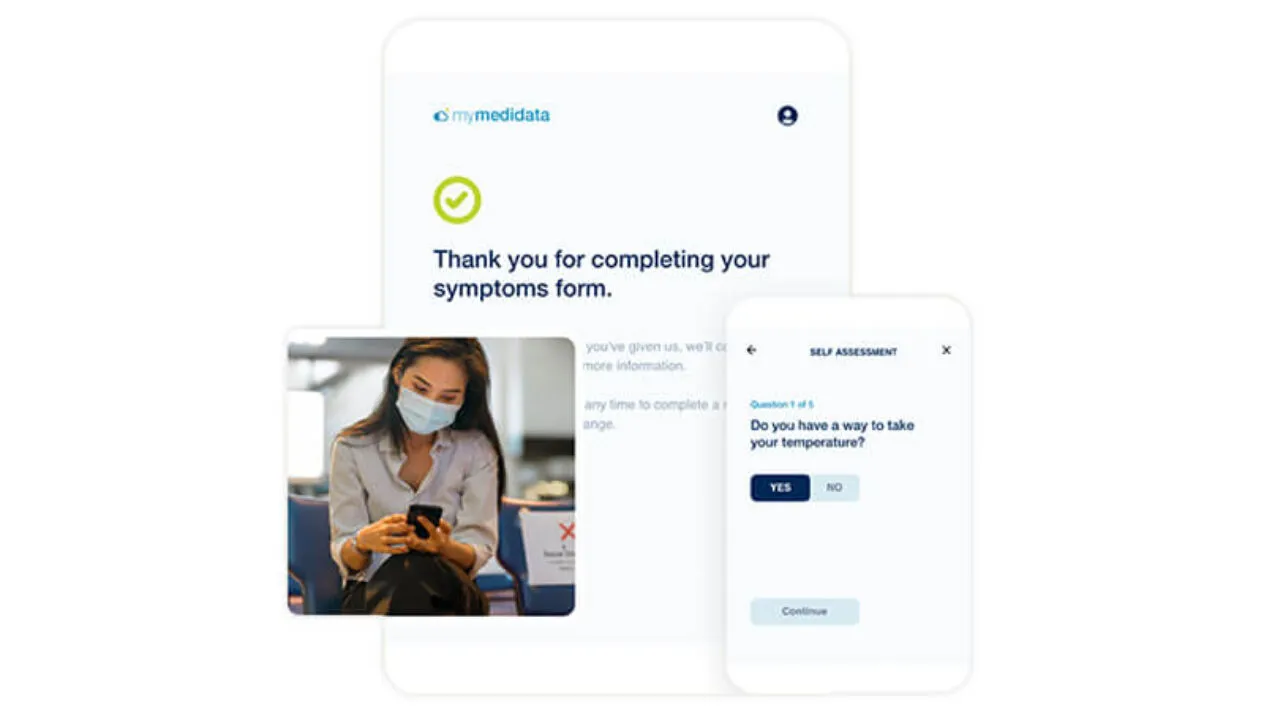
Accelerate Patient Recruitment in Clinical Trials
Accelerate recruitment timelines by building a global community of empowered patients who have already shown an interest in participating in clinical trials.
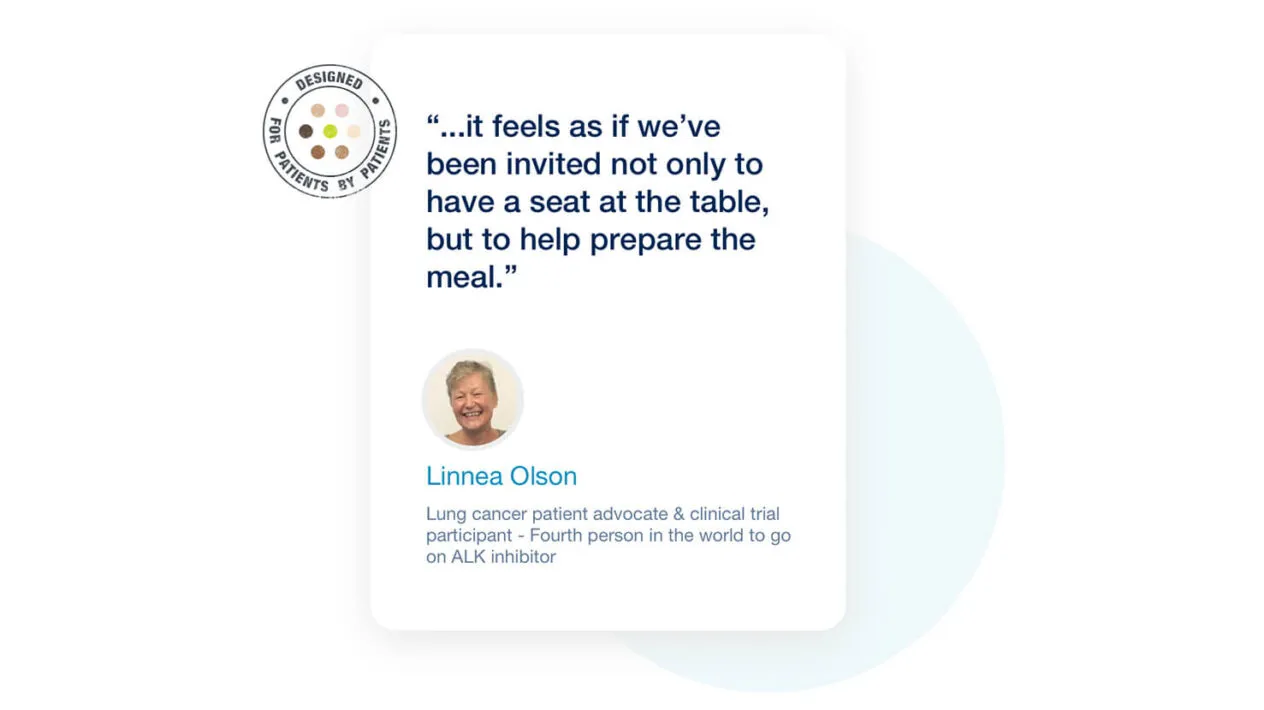
Patient Registries Built for Patients by Patients
myMedidata Registries is designed in partnership with Medidata’s Patient Insights team, a team of dedicated advocates using the award-winning Patient Centricity by Design (PCbD) methodology. Even the most sophisticated innovations in clinical trial technology are meaningless without patient input.To improve patient engagement, Medidata has now expanded access to our Patient Insights Board (PIB) and award-winning Patient Centricity by Design methodology to allow sponsors and CROs to optimize trial design and patient participation.

Enable Cost-Effective and Site-Efficient Patient Recruitment with MD Prescreen
MD Prescreen (MDPS) is Medidata’s recruitment concierge service provided in partnership with Circuit Clinical. Added as an enhancement to myMedidata Registries, MDPS provides additional patient pre-screening and trial matching by qualified nurses and healthcare professionals, ultimately improving the recruitment process for patients, sites, and sponsors.
Related Solutions
Learn More

myMedidata Registries Fact Sheet
Learn how myMedidata Registries helps you accelerate clinical trial recruitment timelines by building a global community of patients who have already shown an interest in participating in clinical trials.
Transforming The Patient Experience with myMedidata Registries and Circuit Clinical
The traditional model of recruiting and retaining patients study by study in a transactional nature isn’t working. Patients are more than the data they provide to a study and are looking to be engaged in novel and everlasting ways.

Patient Centricity and Virtualizing Technologies in a COVID-19 World
Patient centricity is a growing and important movement within clinical trials, and although an official industry-wide definition is lacking, sponsors often refer to it as adopting a culture that puts patients first. This means embracing processes that enable fewer protocol assessments or involve greater use of virtual patient-facing technologies in clinical trials.