Site Cloud: End of Study (EOS) for Clinical Trials
Transform End of Study Data Exchange
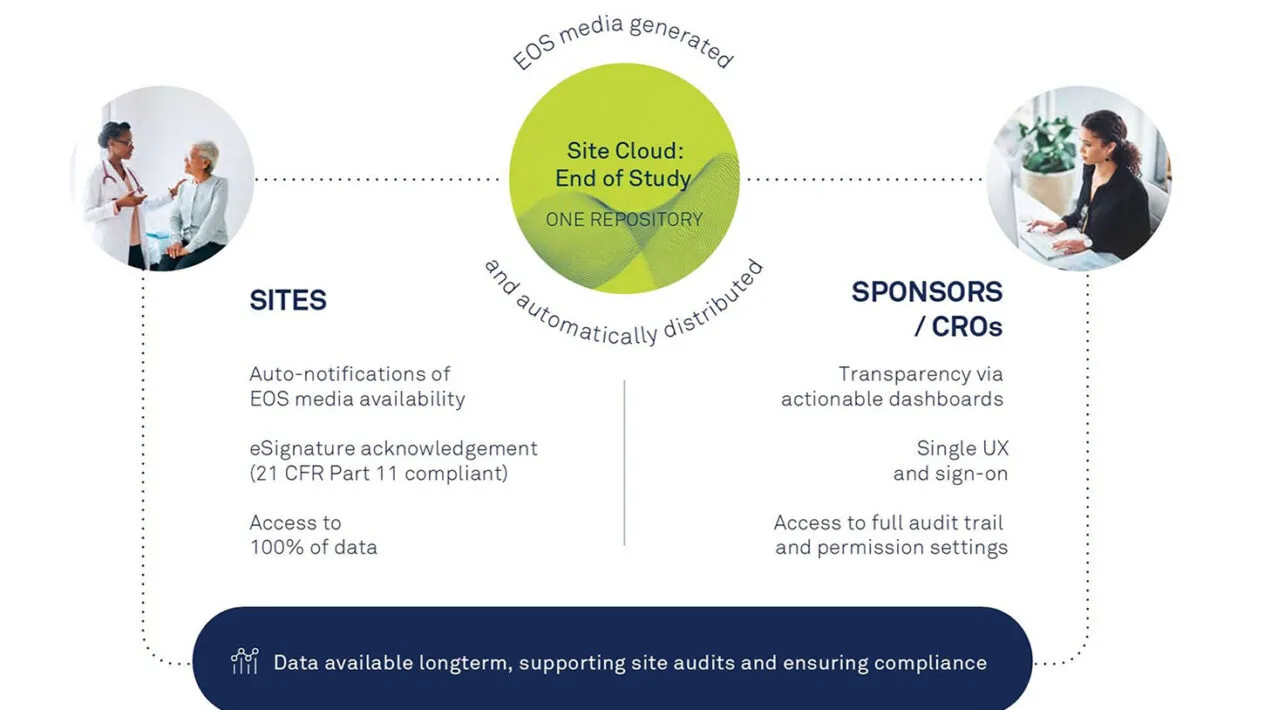
Site Cloud: End of Study (EOS) is the first end-to-end solution that seamlessly generates, distributes, and manages study files at the end of a study.
With EOS, sites’ study files are accessible and downloadable by a secure and trusted unified platform, eliminating the need to create and distribute physical media and deal with manual acknowledgment forms.
Why Choose Site Cloud: End of Study?
Increase Compliance
With controlled access and full audit trail, sites always have access to data. EOS has the highest level of information security, data privacy and quality management.

Reduce Risk
Reduce manual efforts through cloud-based file distribution that eliminates the need to develop and distribute CDs and acknowledgment forms to sites.

Improve Site Sustainability
Reduce site burden by providing a central location for receiving critical study assets.

Eliminate Physical Media
No More CDs! Eliminate risk of damaged media and the burden of providing electronics that read CDs.
Key Features
Automated Generation & Distribution
Take the steps from 15+ to 4 with automation within Site Cloud: End of Study.
Electronic Signature for Acknowledgement
No paper acknowledgement forms. Gain immediate visibility into completion.
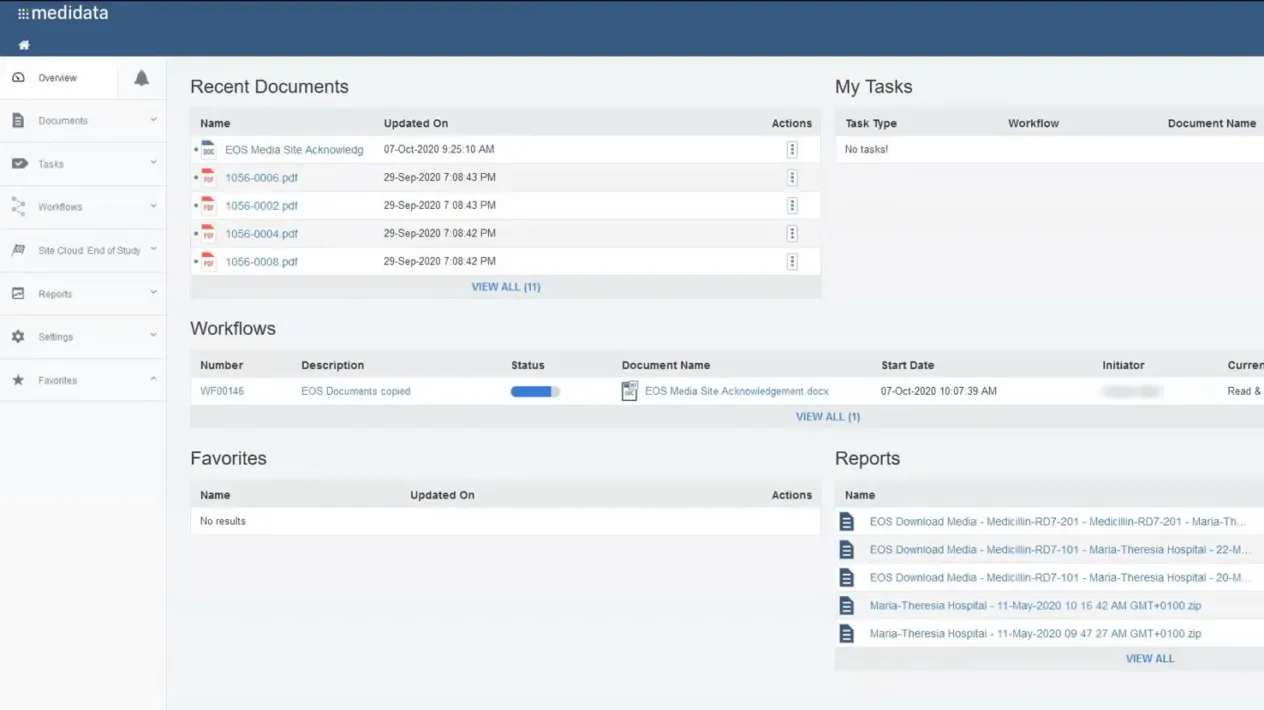
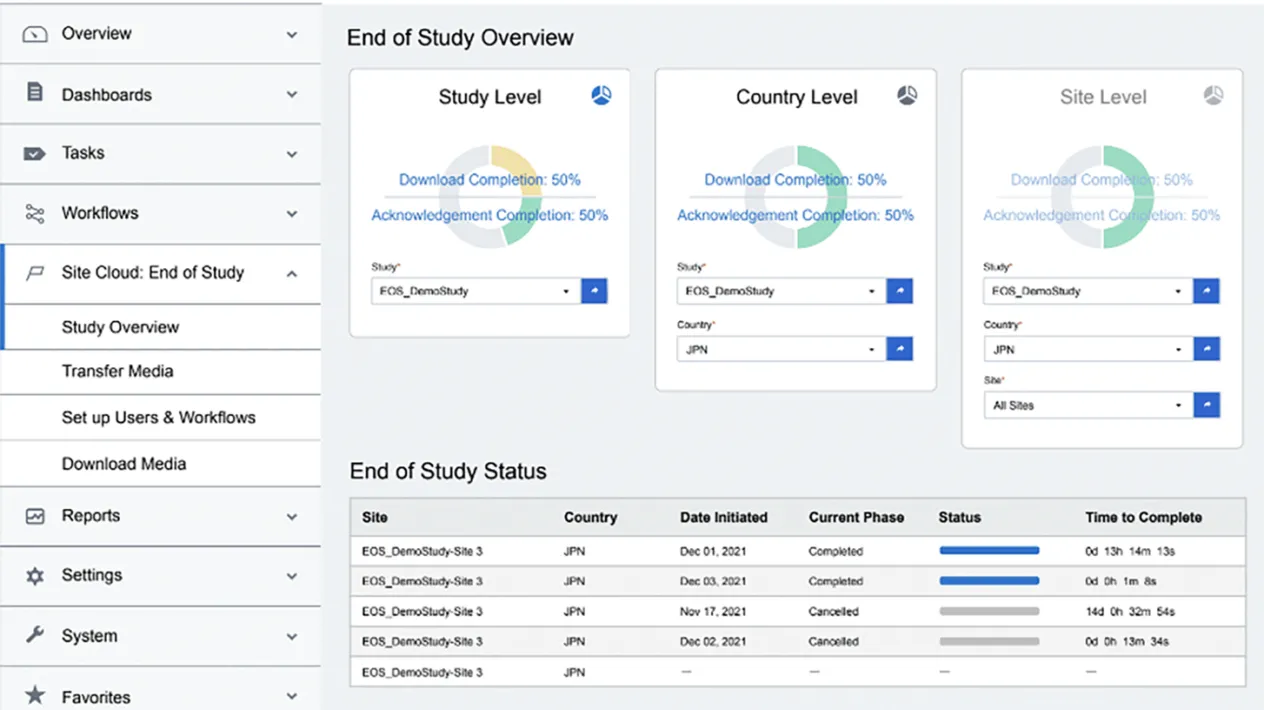
Reporting & Dashboards
Gain transparency into the download and acknowledgement of content along with actionable dashboards.
Full Audit Trail
Track and confidently oversee and report on all EOS document tasks with controlled access and permission settings.
Related Solutions
Learn More

Digitize and Share all End of Study Data
Site Cloud: End of Study is the only platform for digitizing and sharing all end of study data. Explore how you can gain visibility and de-risk end of study data exchange.

Seamlessly Improve Compliance through Study Closeout
Site Cloud: End of Study is the first end-to-end solution that seamlessly generates, distributes & manages sites’ study files at the end of a study. With EOS, sites’ study files are accessible and downloadable via a secure and trusted unified platform, eliminating the need to create and distribute physical media and deal with paper acknowledgment forms.
Digital Transformation: All End of Study Content Has Never Been Easier
The art of closing out a study, and being compliant with regulatory requirements for NDA submission, includes End of Study (EOS) documents that a site must retain. Current business processes to produce, chronicle, and deliver CDs of the requisite EOS Media are manual and time-consuming for sites, CROs, and sponsors. Watch this webinar to hear Medidata’s experts discuss the benefits of utilizing a platform for digitizing and sharing all end-of-study data.
Site Cloud: End of Study Demo
The industry is facing challenges in distributing end of study media. Medidata is transforming the process with automatic availability of all EOS data to both Sponsors and Sites as appropriate, including audit trails, with data available long-term, ready for follow ups and audits.
Watch how Site Cloud: EOS improves operational efficiencies, increases compliance, and improves site satisfaction.