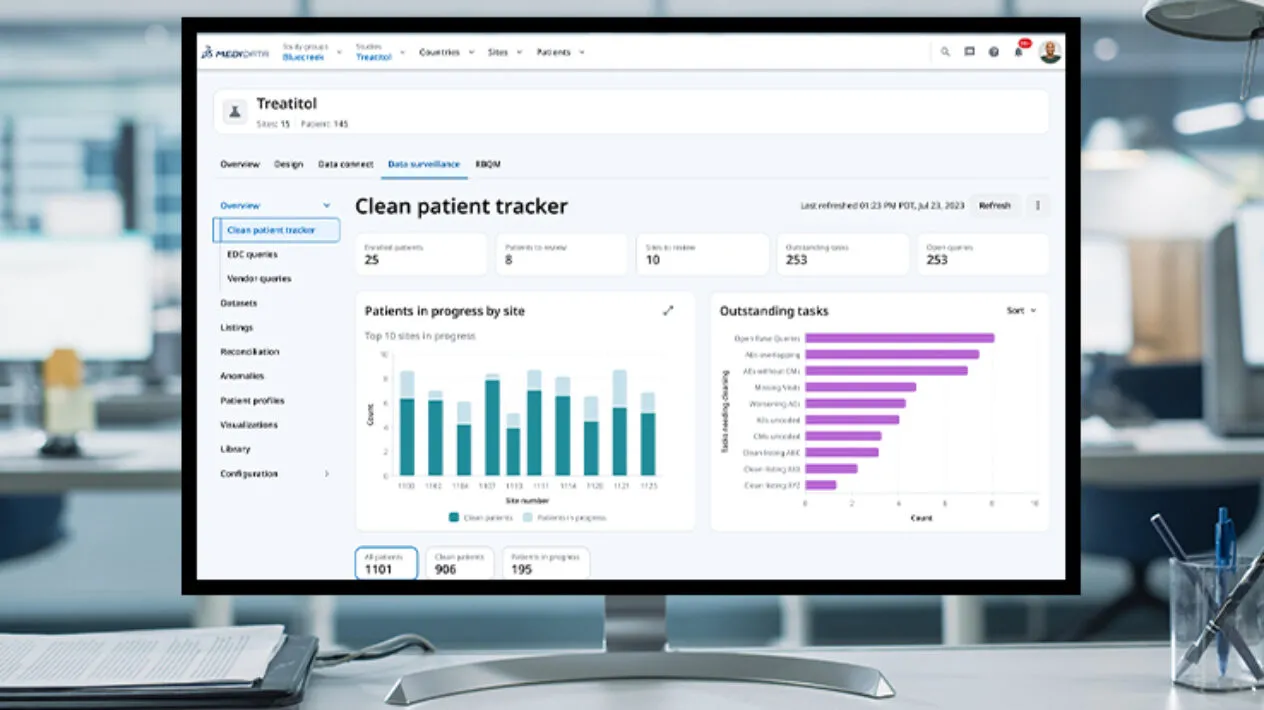
Rave Data Management
Medidata’s Rave Data Management solutions for clinical data management and clinical data capture eliminate complex, manual processes and deliver higher quality data for faster insights. Resulting in critical reductions in study build time, query volume, data correction rates, and reporting turnaround.
Rave Data Management solutions, running on the Medidata Platform, deliver interoperability between the New Medidata Designer, Rave EDC, eCOA, eConsent, myMedidata, Sensor Cloud, RTSM, Imaging, Coder, Safety Gateway and Clinical Data Studio.
“It is always helpful that we’re using multiple modules [from Medidata] and the interface between them is already there. I don’t have to worry about the backend APIs or interfaces between one system and another. It makes it just leaner and easier to use.”
– Salam Ammus, Executive Director, Clinical Data Management, Alkermes

“… Our partnership with Medidata has been very collaborative from a data management perspective… It’s made data management easier for reviewing the data, drawing out metrics, and building the forms. It’s an easier platform to design.”
– Cathy Hult, Director of Data Management, PROMETRIKA

“…the Medidata products are very innovative and support our very complex oncology studies…”
– Vijay Chundru,Senior Director, EDC Programming Team, Global Clinical Data Operations, Jazz Pharmaceuticals

Clinical Data Capture and Management Demo
See the Medidata Platform’s clinical data capture and management capabilities in action. Includes eConsent, eCOA, RTSM, Sensors, Imaging, and capture of EHR data; and Clinical Data Studio with no code, drag and drop listings, and automatic generation/posting of multiple queries to enable comprehensive, aggregated data review.
Your Path to Next- Generation Clinical Data Management

Navigating the Frontier of Clinical Trial Data Management: Challenges and Innovations
Listen to a replay of this expert panel from Sanofi, Bayer, Daiichi Sankyo and Medidata as they delve into the dynamic clinical trial data management landscape. You will learn about the latest approaches and cutting-edge technologies that are now available to improve drug development and patient outcomes.
Accelerate Data Integration, Standardization, and Review
With Medidata Clinical Data Studio, Rave EDC data, other Medidata data sources, and even non-Medidata data can be easily integrated, standardized, reviewed, and reconciled in a no/low-code environment powered by AI.
Empowering The Transformation From Clinical Data Management To Clinical Data Science: We Have The Technology!
As the clinical trial data acquisition landscape expands, the industry requires new approaches to support the rapid pace and increasing diversity of data sources. Thus, it’s not surprising that the focus of clinical data management teams is shifting from reactive, exhaustive data cleaning to proactive, risk-based clinical data science.
Learn how Medidata empowers clinical data managers to transform into clinical data scientists and become more efficient, effective, and expert.
Risk Proofing Decision-Making
Protocol requirements often involve multiple data points from different data sources to determine screening criteria and calculate endpoint measurements. Multiple methods of data collection from sources like EDC, RTSM, eCOA, and Imaging increase data silos making it harder for site staff and study teams to access clean, reliable data and reducing the ability to make effective decisions This recorded presentation uses typical protocol requirements and illustrate the value of using the Medidata Platform to collect and aggregate data to drive key study conduct decisions such as screening, open-label extensions, and cycle decisions.
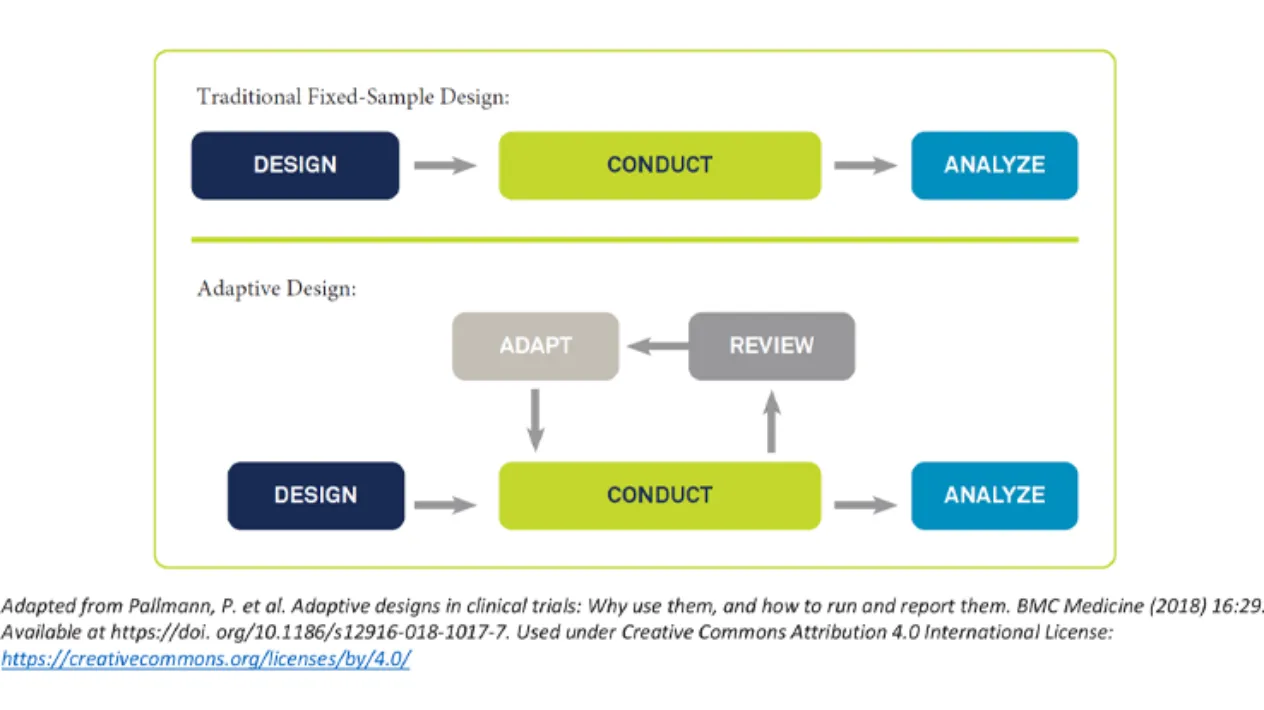
Adaptive Trials
Adaptive designs leverage accumulating results during a study to adapt the course of the trial and benefit both early and confirmatory studies.
Medidata has extensive experience with adaptive trial designs. Our multidisciplinary expert teams can help with the planning and implementation of your adaptive design, so that common challenges and pitfalls are circumvented.
Benefits of Rave Data Management

Reduced Risk
Reduce risk to data integrity, data quality and patient safety. The Medidata Platform delivers complete data transparency within a study or across a portfolio, empowering study teams to accelerate decision-making and drive study efficiencies, ultimately reducing risk and timelines.
Accelerated Timelines
Managing all your data on a single, unified platform improves pace and productivity.
The Medidata Platform offers streamlined workflows enabling faster study build and database lock.
Agility and Flexibility
Medidata’s platform meets the agility and flexibility needs of any trial. Medidata and our customers perform an average of over 30,000 mid-study changes per year, including for studies with tens of thousands of patients. The combination of our technology and experienced Professional Services team ensures smooth implementation of mid-study changes, with minimal downtime, for even the most complex, large-scale, and time-critical trials.

Simplified Integration
The Medidata Platform unified platform delivers full interoperability across our applications. It enables you to reduce redundancy and non-value added activities derived from data transfers, integrations and reconciliations common with disparate systems.
Medidata also has an open integration framework and APIs enabling integration with any in-house or 3rd party system.

A Guide to Leveraging AI Strategically
This guide breaks down the “Five Es” of AI and explores how AI can solve important problems, enhance processes, improve user experience, demystify AI’s “black box,” and uphold ethical standards. It shares the best paths for organizations to drive the adoption of AI in clinical data management.
Products
Rave EDC
Rave EDC is the most advanced, robust, and secure system for clinical trial site, patient, and lab data capture and management.
Clinical Data Studio
Accelerate clinical data integration, standardization, review, and reconciliation with Clinical Data Studio.
Rave RTSM
Rave RTSM is the only fully pre-validated randomization and trial supply management solution that can be configured in minutes and enables mid-study changes with minimal downtime and no change orders.
Medidata eCOA
Medidata eCOA (Clinical Outcome Assessment) is revolutionizing the way sponsors, CROs, and sites collect electronic data from patients, physicians, and caregivers.
Medidata eConsent
Medidata’s eConsent is an innovative, regulatory-compliant, patient-friendly, electronic consent system for clinical trials.
Medidata Sensor Cloud
Medidata Sensor Cloud takes a unique approach to the ingestion, standardization and scalability of data from any commercial or medical grade sensor device.
myMedidata
myMedidata is a single-destination patient portal, enabling patients to virtually enroll and participate in clinical trial activities.
Rave Imaging
Rave Imaging provides cloud-based, secure management for all your medical imaging tasks in an innovative and intuitive system.
Rave Coder
Rave Coder provides medical coding for verbatim terms from Rave EDC and external sources using the MedDRA, WHODrug and JDrug dictionaries.
Rave Safety Gateway
Rave Safety Gateway delivers precise, accurate, and efficient transmission of AEs and SAEs in Rave EDC to your safety system.
Site Cloud End of Study
Site Cloud End of Study (EOS) is the first end-to-end solution that seamlessly generates, distributes & manages study files at the end of a study.
Learn More
Revolutionizing Clinical Studies with Adaptive Trial Designs
Medidata has extensive experience with adaptive trial designs. Our multidisciplinary expert teams can help with the planning and implementation of your adaptive design, so that common challenges and pitfalls are circumvented. Discover the benefits of adaptive clinical trial designs. Maximize outcomes, efficacy, and patient safety.