Powering Decentralized Clinical Trials with Medidata and Circuit Clinical
Delivering on the promise of hybrid or fully decentralized clinical trials requires proven expertise that meets the needs of patients, sites, and sponsors.
Medidata and Circuit Clinical provide a turnkey solution that enhances patient access and inclusion through site ratings and a network of decentralized sites – all trained and certified on Medidata technology.
Transforming Clinical Trials for Patients, Sponsors, and Sites

Accelerate Business and Clinical Goals
The demand for decentralized clinical trials requires a turnkey approach that can scale to meet your needs. With Circuit Clinical, ready-made DCT sites are standardized on Medidata’s Rave and Patient Cloud solutions, making Medidata the only partner able to deliver a complete DCT solution.
Key Features

Established Decentralized Clinical Trial Network
Leverage Circuit Clinical’s network, standardized on Rave and Patient Cloud technology solutions, representing over 90 doctors, across 30+ site locations, and a nationally accredited cancer center with a database of more than 2.5 million patients who may qualify for clinical trials.

Enable Cost-Effective and Site-Efficient Referrals for Patient Recruitment with MD Prescreen
MD Prescreen (MDPS) is Medidata’s recruitment concierge service provided in partnership with Circuit Clinical. Added as an enhancement to myMedidata Registries, MDPS provides additional patient pre-screening and trial matching by qualified nurses and healthcare professionals, ultimately improving the recruitment process for patients, sites, and sponsors.

Single Destination Patient Portal
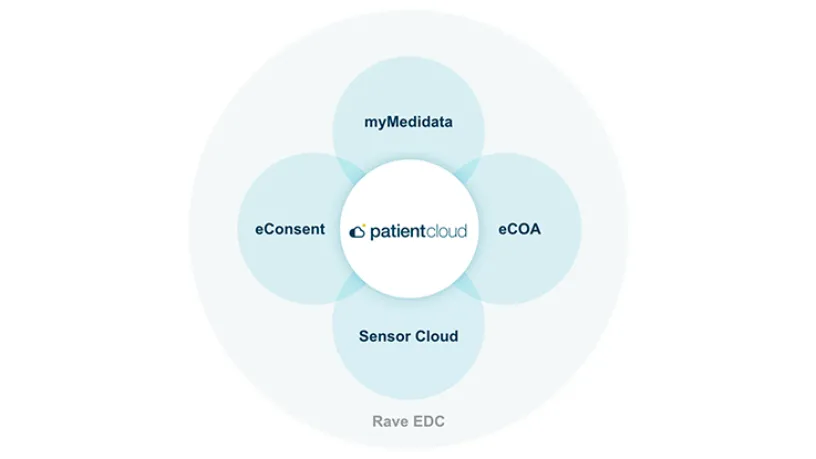
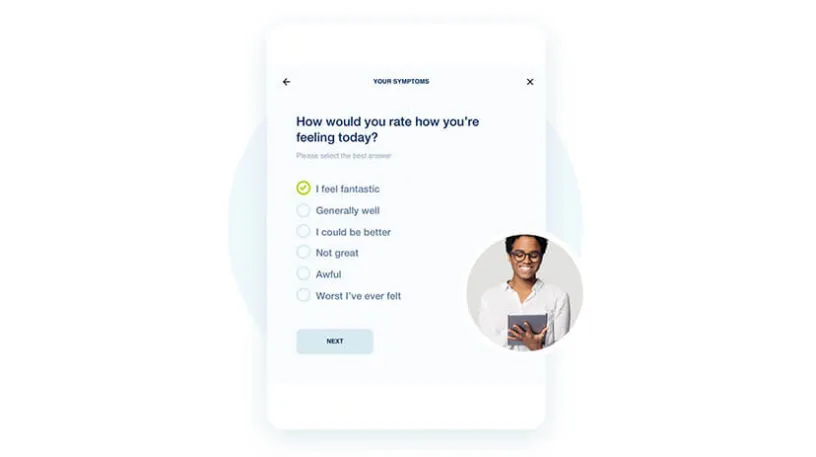
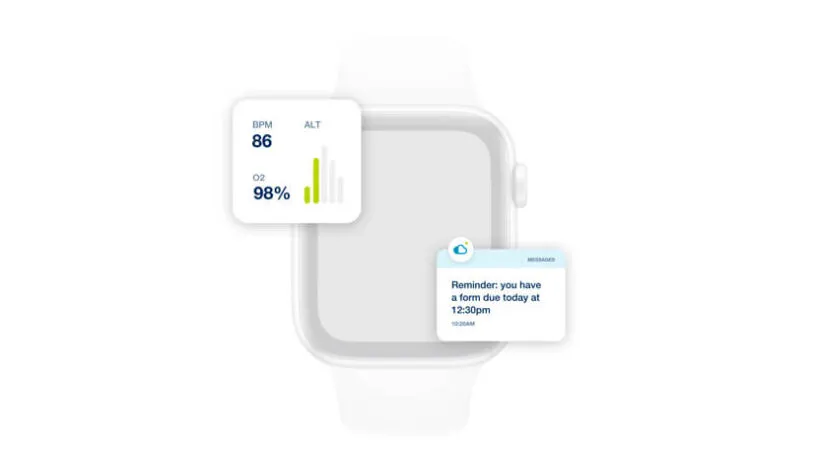
With myMedidata, patients can use any online device to learn, enroll, and participate in clinical trial activities, offering a streamlined and trusted approach to participation.
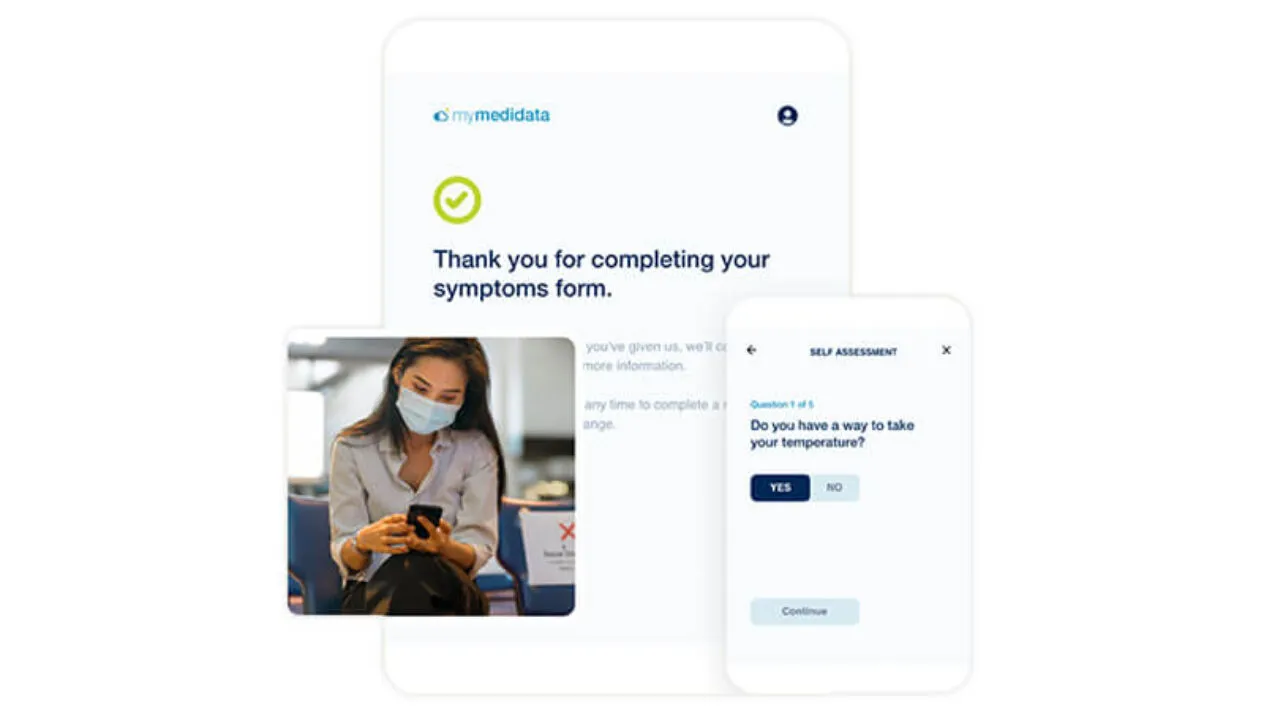
Expand Patient Participation in Clinical Trials
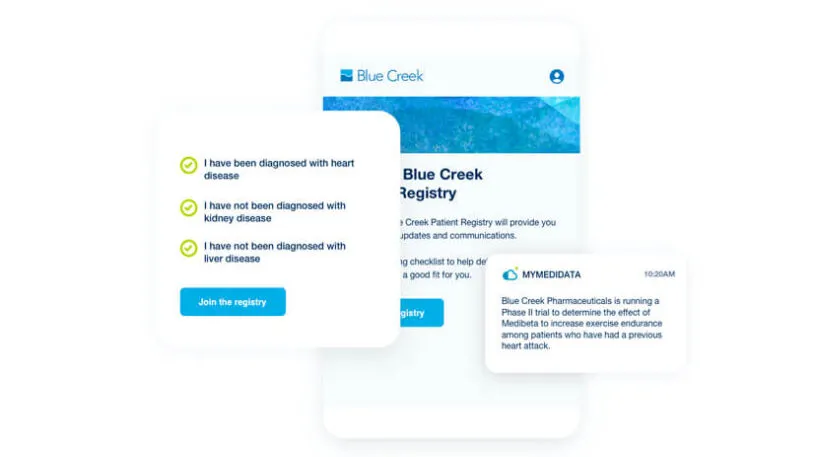
myMedidata Registries expands patient participation from a single trial transaction to pre-and post-trial engagement, resulting in a community of educated, empowered and engaged patients.
Related Solutions
Learn More
Create a better patient experience and transform clinical trials with myMedidata
See how myMedidata brings patient-facing solutions into one unified platform, giving sites the ability to streamline operations and maintain a consistent and traceable process, built using input from Medidata’s Patient Insights team of patient advocates.

Struggling with patient enrollment and retention? Unsure how to enable virtualization in your clinical trials?
See how myMedidata can improve patient engagement and retention, accelerate clinical trial timelines, and mitigate risks with study virtualization.

Patient Centricity and Virtualizing Technologies in a COVID-19 World
Measuring the pandemic’s impact on driving adoption of digital tools in fully virtual, hybrid, and traditional clinical trials.

Accelerate Clinical Trial Recruitment Timelines
Learn how myMedidata Registries helps you accelerate trial recruitment timelines by building a global community of patients who have already shown an interest in participating in clinical trials.