Medidata Consent
Enrollment Simplified
Medidata Consent, part of the Medidata Patient Experience, is an innovative, patient-friendly electronic informed consent and patient enrollment system. Using multimedia technology, your patients are educated and guided through understanding key elements of a clinical trial.
Consent empowers sites to conveniently inform and consent patients through the Medidata App, the industry’s only comprehensive site-facing portal, which streamlines clinical site operations, enhances study efficiency, and empowers patient involvement. Consent provides patients, sites, CROs, and sponsors with a unified enrollment experience while delivering additional study analytics to the study team to understand trends and insights from the consenting and reconsenting process.
Industry-Leading Consent Experience
Proven at scale with the expertise, global reach, and regulatory-ready consent solution to drive your clinical trial success.
Patients
Sites
Studies
IRB/ECs
Faster Consent Build Time
Countries
The Value of Medidata Consent

Greater Efficiency
Medidata Consent is designed to support even the most intricate study designs, accommodating pediatric trials, complex multi-arm trials, and simpler scenarios through a scalable platform with easy-to-use configurations versus customization.

Create a Better Patient Experience
Medidata Consent advances patient compliance and retention while enabling an increased understanding of study objectives, risks, benefits, and responsibilities. Our dedicated Patient Experience Helpdesk optimizes the site and patient experience when and where you need it most.

Increased Transparency
With Medidata Consent, sponsors can view enrollment progress at specific sites and across their trials while monitors can track enrollment in real time, enabling remote monitoring and saving travel costs.

Enhanced Site Productivity
Medidata Consent, part of the Medidata Patient Experience, comes unified with the Medidata Platform, delivering a familiar environment that reduces friction and improves adoption.
Key Features of Medidata Consent
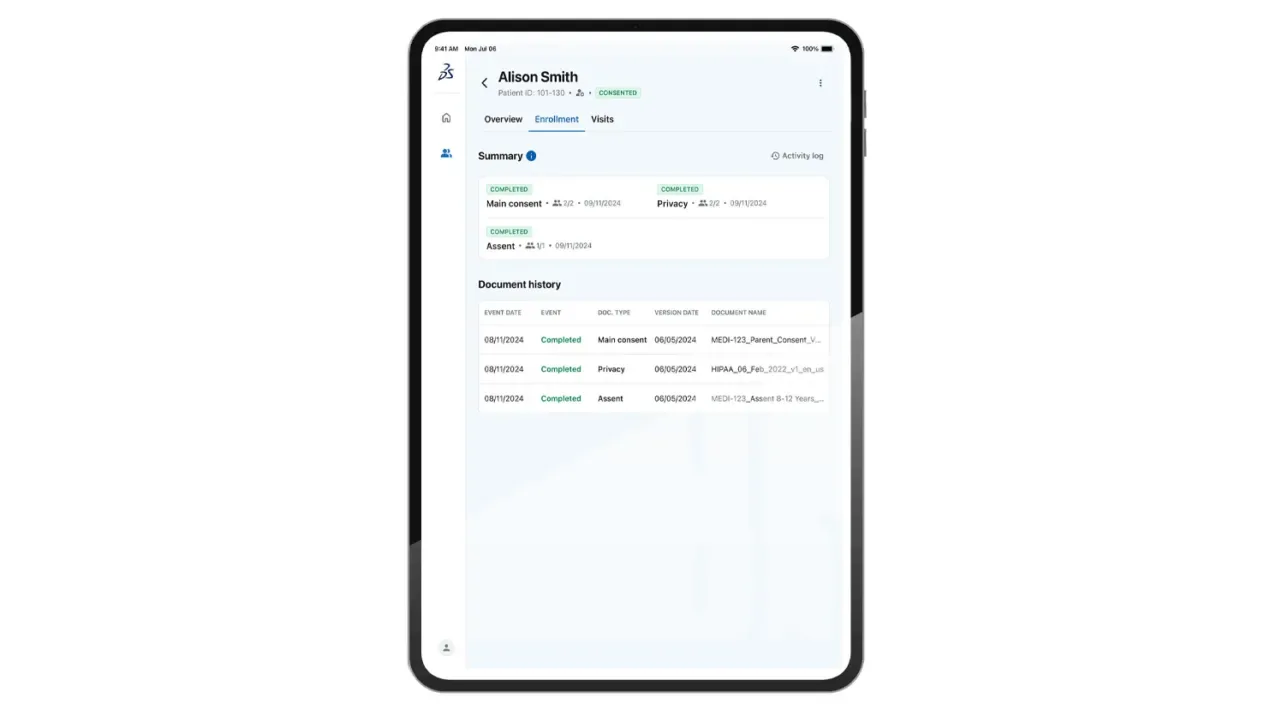
Consent is unified with study build and site operations tools, streamlining the setup and management of study settings and assets from a single interface.

Medidata Consent simplifies the complexities of pediatric trials through age adaptive workflows and videos.

Built with the ultimate flexibility, clinicians can personalize consent workflows to tailor required and optional documents for each patient.

Dedicated teams of experts to ensure you realize the most value for your investment through comprehensive support for sites, sponsors, and patients.
Learn More

The Use of Electronic Informed Consent (Consent) in a Blood Collection Study
A specialty division of a top ten pharma was preparing for an upcoming blood collection study, looking to recruit 6,000 patients spanning ten sites.
Moving away from their paper process, Medidata Consent modernized the clinical trial consenting process for patients, sites, and sponsors. Learn more about how Medidata Consent improved patient comprehension and reduced site workload.

Simple Enrollment
Medidata’s Consent is an innovative, patient-friendly, electronic informed consent and patient enrollment system for clinical trials. Built with the ultimate flexibility in mind, patients can choose to provide consent at the site or remotely depending upon the design of the study.

Electronic Informed Consent in Clinical Research
This white paper provides an overview of findings from the Medidata Consent study to understand the regulatory positions, adoption, and the variability regarding Consent globally.

Why Paper Consent Is Your Bottleneck in eCOA Clinical Trials
In clinical trials that use electronic clinical outcome assessments (eCOA), the paper-based consent process can be inconsistent with modern digital standards.