Bridging the Gap – TEFCA, Healthcare Data Interoperability, and Clinical Research Use Cases

Accessing data from multiple electronic health record (EHR) systems results in challenges caused by duplication, gaps, or inconsistencies in data. The lack of standards and interoperability between EHRs and other systems in the healthcare ecosystem can have dire consequences. For example, critical information from a patient’s medical history might not be visible to hospital staff due to a lack of integration with other systems. That missing data could lead to an adverse event, which in turn could lead to a patient’s death.
The issues are by no means insignificant. In the US alone, the lack of interoperability has an estimated cost of $30 billion per annum (figure 1), much of which is avoidable.
Figure 1. What happens when we don’t have an interoperable healthcare system?
These issues have plagued the industry for decades. So, it’s no surprise that key questions asked are: why is this still an issue, and what’s being done to improve matters?
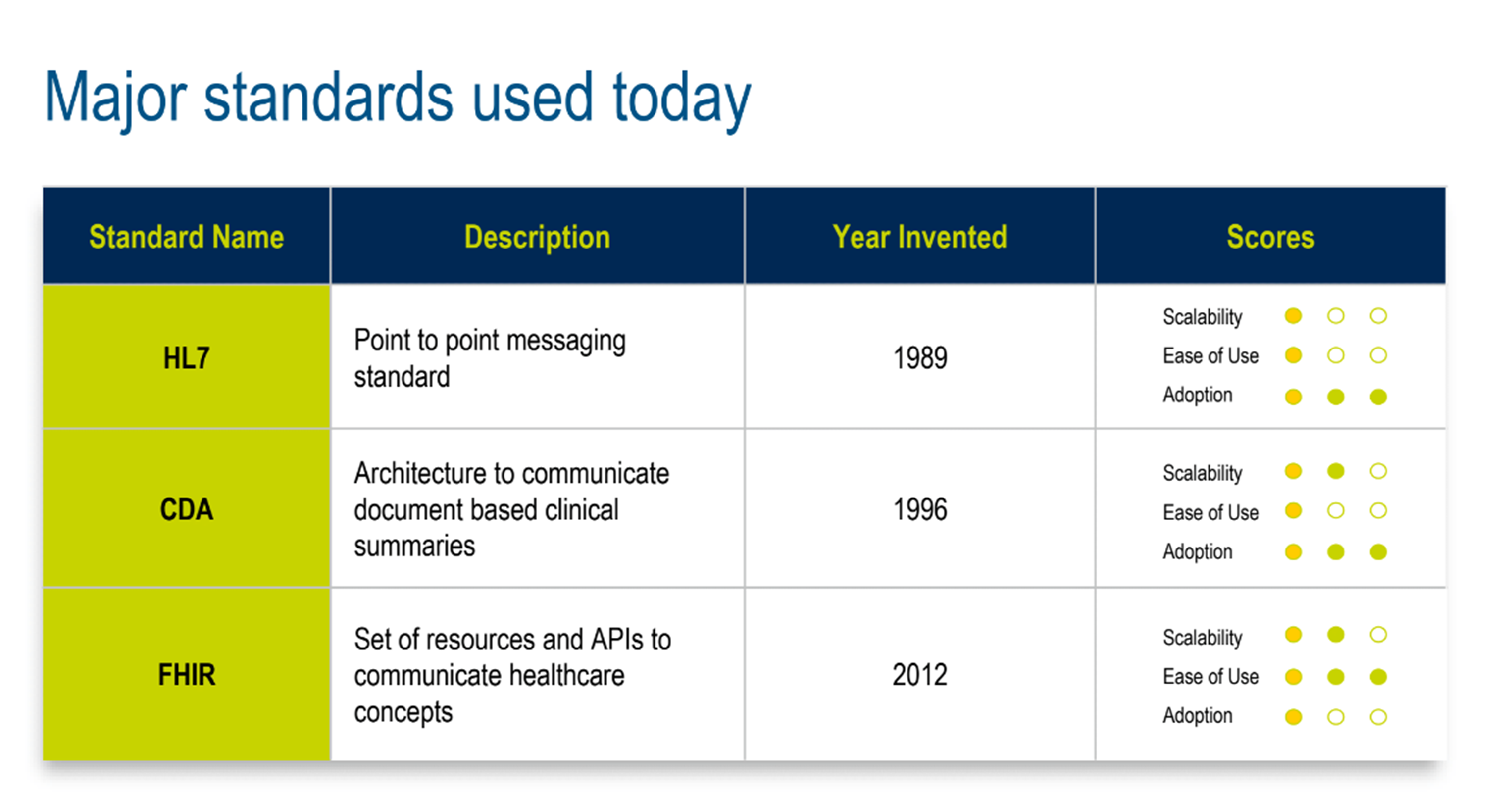
In a joint presentation, Dan Braga (VP, Healthcare and EHR Solutions, Medidata) and Samir Jain (Sr Director, Product Management, EHR Solutions, Medidata) summarized the pros and cons of historical standards (figure 2) that are still in use today—from the early days of systems “within four walls”, to point-to-point data sharing, to the web standards of today.
Figure 2. Major standards that are used today.
Clearly, with a continued yearly excess cost of $30 billion in the US alone, attempts to address and standardize interoperability-related problems have had limited success. In our presentation, we shared an overview and use cases of the three core challenges faced by the industry (figure 3): ambiguity and flexibility in data standards, problems related to point-to-point connections, or multiple networks, and the need for data-sharing agreements and trust frameworks.
Figure 3. Where does that leave us?
With these challenges in mind, we were excited to share news of the industry initiative that will lay the foundation for an industry-wide transformation, where systems interoperate and share data using gateways and networks.
As part of the 21st Century Cures Act, the Office of the National Coordinator for Health Information Technology (ONC) was mandated to create an “interoperability floor” for clinical data exchange across the US. This initiative is called the Trusted Exchange Framework and Common Agreement (TEFCA) (figure 4). The Trusted Exchange Format (TEF) refers to the technical standards for exchange. The Common Agreement (CA) relates to data-sharing agreements that allow for open sharing of data within the network.
Figure 4. What is TEFCA?
The initial stage of TEFCA is focused on treatment use cases, but we expect to see clinical research addressed in the future.
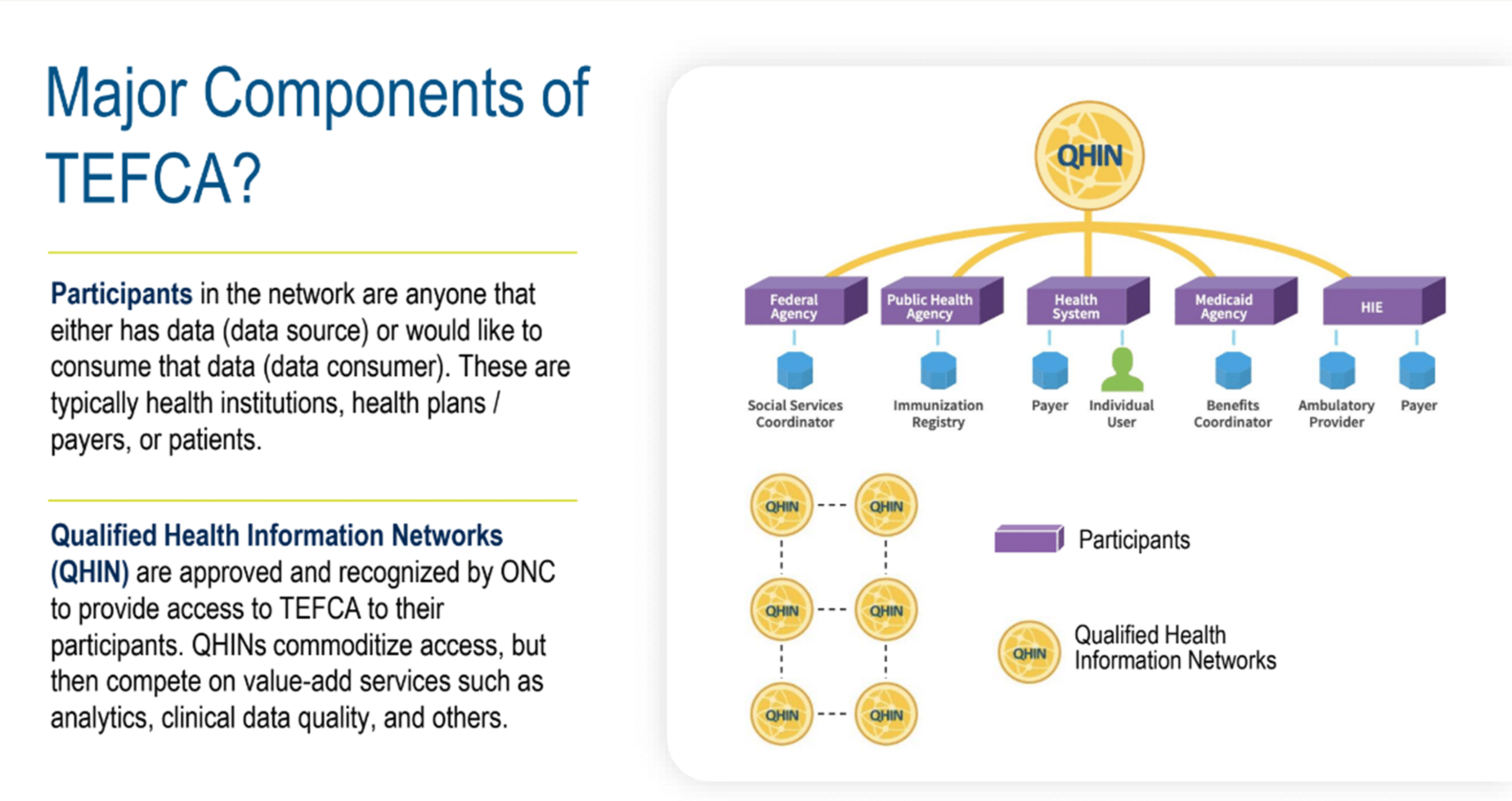
The framework is a hub and spoke concept; connect once with a hub, then interoperate with everyone in that network (figure 5). The hubs would standardize data output and manage the inbound and outbound connections, so stakeholders, such as sponsors, CROs, sites, and technology providers, wouldn’t need to do that themselves.
Figure 5. Major components of TEFCA.
Hub and spoke network concepts are known to be highly scalable, efficient, more manageable, and significantly faster to implement and integrate. To put that into context, think about setting up five hundred individual point-to-point connections, then compare that to connecting once to a single hub with five hundred connections.
As part of this initiative, to create a “network of networks” for sharing health data across the US, the first six potential hubs (figure 6), called Qualified Health Information Networks (QHINs), were announced by the U.S. Department of Health & Human Services on February 13, 2023. The companies are Kno2, Health Gorilla, eHealth Exchange, Epic, Commonwell Health Alliance, and Konza.
Figure 6. Current TEFCA QHINs.
On December 12, 2023, an announcement confirmed that eHealth Exchange, Epic Nexus, Health Gorilla, KONZA, and MedAllies had been officially designed as QHINs. Kno2 announced they met the QHIN requirements in February 2024.
As EHR data is increasingly utilized across the clinical trial ecosystem, the impact of a lack of interoperability and integration has long been felt across the entire healthcare and clinical trial ecosystem. Leveraging TEFCA will have a significantly positive effect.
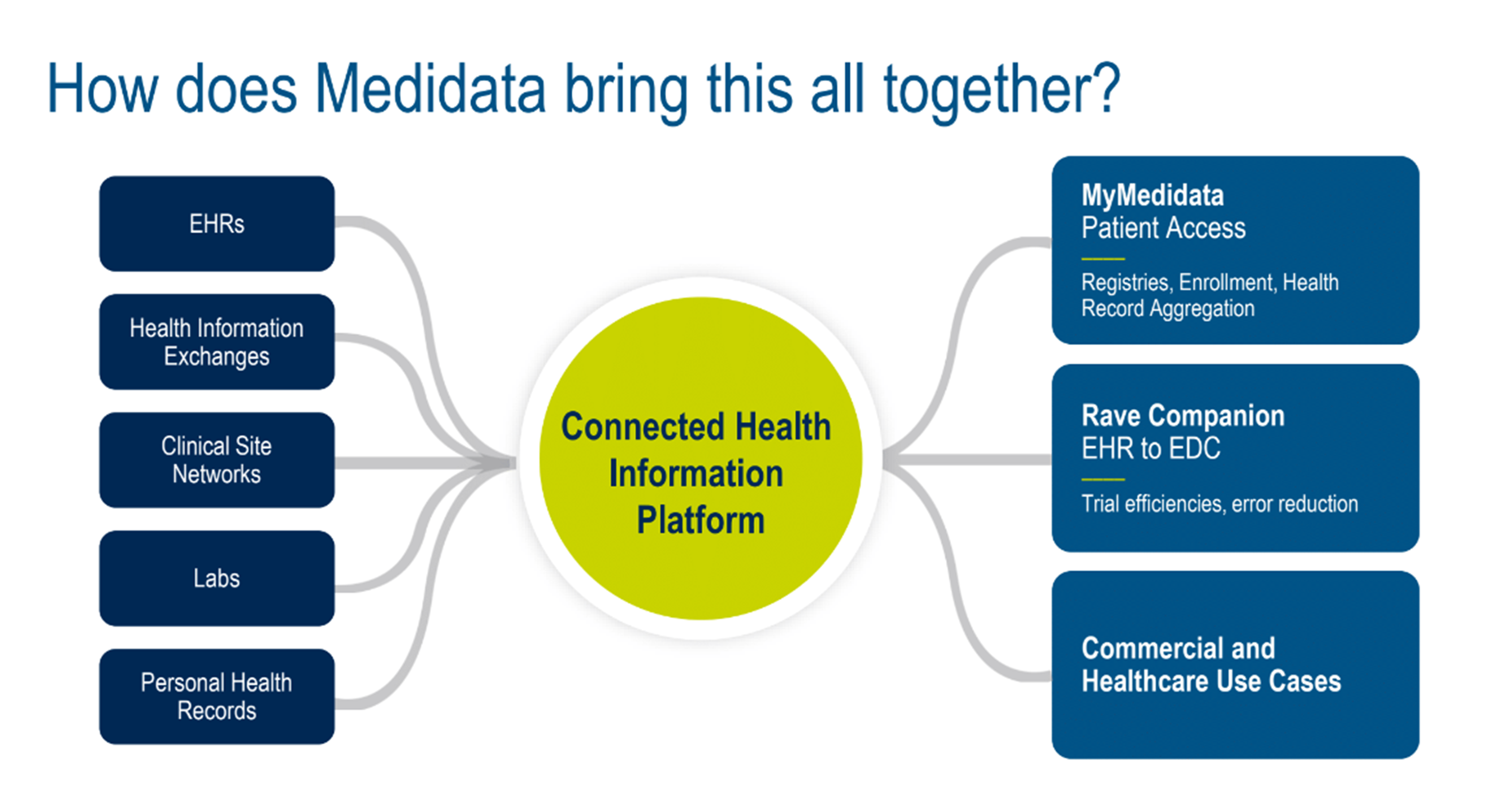
At Medidata, we're working on a number of use cases made possible by leveraging TEFCA with our existing solutions—the myMedidata Patient Portal and Rave Companion. We also discussed other commercial and healthcare examples (figures 7 and 8).
Figure 7. How does Medidata bring this all together?
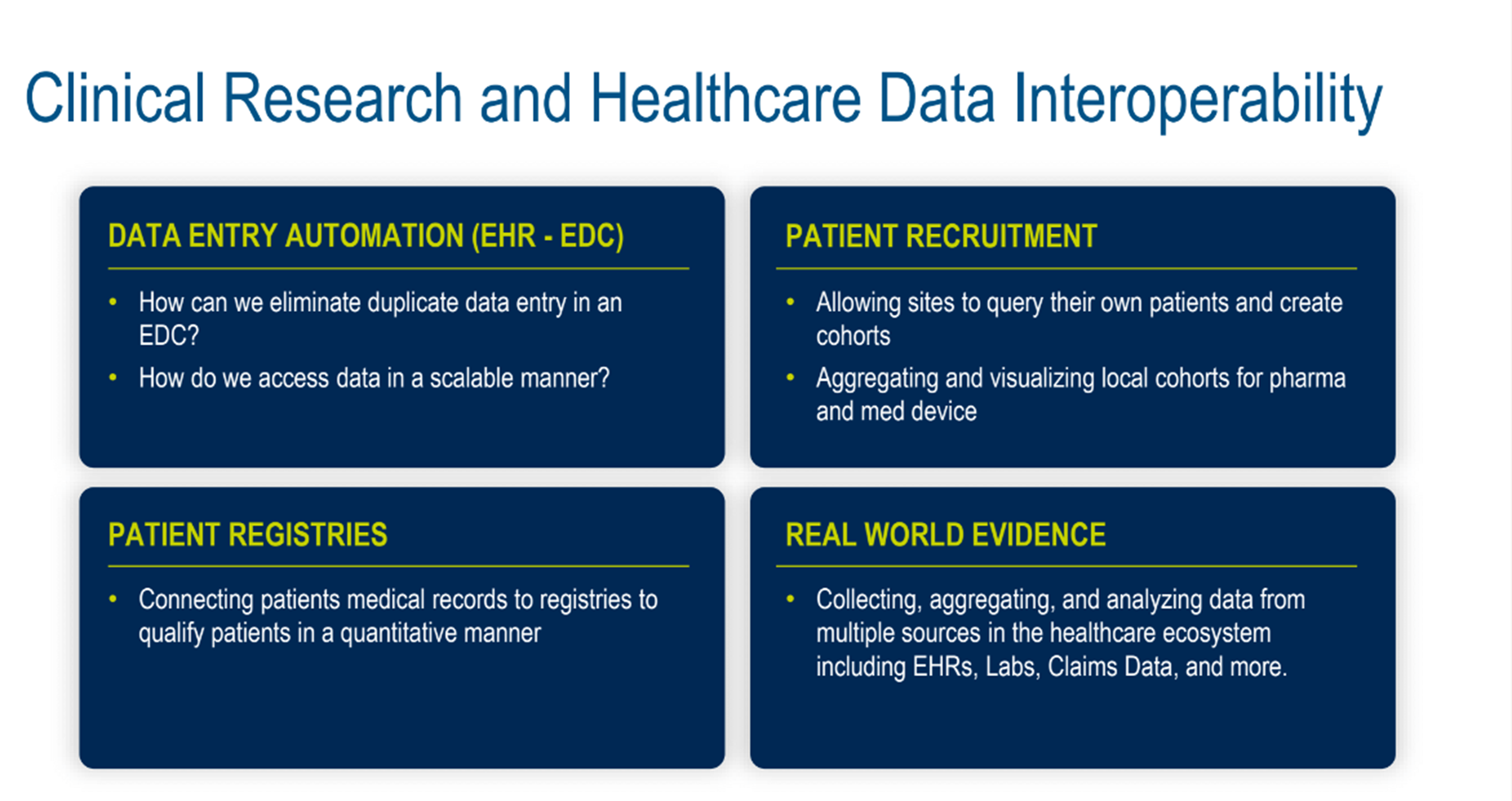
Figure 8. Clinical research and healthcare data interoperability.
The examples we chose touched on data entry automation (EHR-EDC, eliminating duplicate data entry in an EDC solution, and accessing data in a scalable manner), patient registries (connecting patients’ medical records to registries to qualify patients quantitatively), and patient recruitment (allowing sites to query their patients and create cohorts). We also discussed aggregating and visualizing local cohorts for pharma and medical device studies and real-world evidence (collecting, aggregating, and analyzing data from multiple sources in the healthcare ecosystem, including EHRs, labs, claims data, and more).
The message is clear: truly bridging the gap between healthcare data and clinical research data is within reach.
Watch the presentation here to learn more about this industry-changing initiative and how Medidata is taking full advantage of its opportunities to transform clinical research.
To learn more about Medidata’s technology for secure and compliant access to healthcare data, visit our Medidata Health Record Connect page.
Explore Related Articles
Contact Us