Centralized Monitoring: A 360° Approach to Clinical Oversight

The clinical trial industry is facing several challenges today. Increased protocol complexity makes it harder to enlist sites that can deliver patients and high-quality data. Patient retention remains an obstacle despite the flexibility provided by remote and decentralized data collection. The variability of data sources and an increased number of stakeholders are creating a significant operational challenge. And lastly, navigating the ever-changing landscape of global regulatory guidelines without clear direction adds to the difficulties.
So how do we address these challenges?
A risk-based quality management (RBQM) framework, enabling a holistic risk-based quality management and monitoring approach from protocol development to submission, is the backbone that connects stakeholders and processes to ensure collaboration, efficient processes, better data quality, and patient safety oversight. But a framework without technology is just theory—useful in thought, although less impactful.
To address these issues, the industry must embrace a risk-based approach and innovation that allows for real-time access to all data, historical data insights, and tap into automation and AI to achieve comprehensive data monitoring.
The Clinical Trial Ecosystem Challenges
Increased Protocol and Regulatory Complexity
As clinical trials grow more complex in design and execution, it becomes more challenging to optimize their performance and maintain protocol adherence. Additionally, navigating fast-evolving global regulatory guidelines proves even more difficult. Regulators often provide high-level directions that are vague and open to interpretation, creating uncertainty and leading to more stringent, traditional execution methods “just to play it safe.”
Site Selection, Patient Recruitment, and Retention
The pool of experienced investigators to participate in research or enroll patients is shrinking as the clinical research ecosystem grows more complex. And site selection has historically relied on less robust data regarding site performance, patient populations, and investigator experience. This creates challenges in selecting optimal sites that can enroll sufficient patients and deliver quality data. Patient enrollment continues to be a hurdle due to the competitive landscape, lack of awareness, and logistical barriers, particularly hindering the enrollment of diverse populations. Patient retention is further complicated by overall patient burden, disengagement, and a perceived lack of benefit.
Massive Amounts of Data
As data volume and velocity increase, especially with electronic health records (EHR) and electronic medical records (EMR), continuous data from sensors, or unstructured data like images, it becomes difficult to seamlessly process, integrate, and analyze everything in one place. Metadata from various systems and sources is becoming a first-class citizen in the overall data ecosystem, further complicating data cleaning approaches.
A Disconnected Ecosystem
Sponsors, CROs, and sites are often juggling a patchwork of specialized, standalone technologies that are disconnected and do not communicate with each other. The result is duplicated efforts, delays, and fragmented data spread across multiple systems, making it more difficult to manage. Traditional siloed processes fall short in handling today’s complex, data-rich studies, often leading to delayed insights, extended timelines, and increased costs. Maintaining oversight and ensuring consistent protocol adherence becomes difficult without a unified system.
More Stakeholders
Historically, patients were primarily viewed as research subjects. Today, there's a growing recognition that patients are crucial partners. Their lived experiences, preferences, and feedback are increasingly valued in study design, protocol development, recruitment strategies, and even the dissemination of results. This shift has given rise to new stakeholders, such as patient advocacy groups, individual patient advocates/advisors, and caregivers and family members.
Beyond traditional service providers like CROs, we've seen the rise of specialized niche vendors that should be included in all phases of planning and collaboration.
As real-world evidence (RWE) gains traction, the role of everyday clinicians and healthcare systems in providing de-identified patient data for research becomes more significant, making healthcare providers (beyond the primary investigator) vital stakeholders.
RBQM Adoption
Despite its well-established benefits, RBQM adoption remains lower than expected, although adoption is increasing—especially around initial risk assessment and somewhat centralized monitoring. An industry survey revealed that 88% of clinical trials included at least one RBM or RBQM component in 2023, up from 53% in 2019. But challenges persist, including a lack of awareness, knowledge, skills, and technology.
The 360° Monitoring Framework
IMAGINE is a 360° monitoring framework that addresses many of these challenges by providing an integrated, automated approach that leverages all available data to inform study design, assess what’s critical, and enable cross-stakeholder workflows, collaboration, better planning, and adaptive risk monitoring (Figure 1).
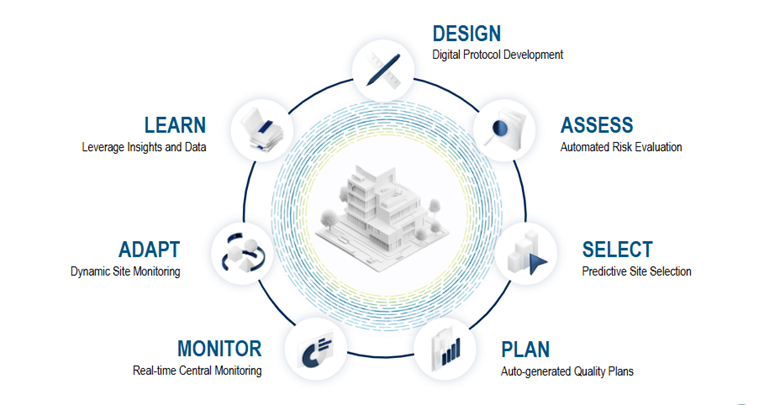
Transforming Clinical Trials: How a Digitized Protocol Drives Efficiency and Accuracy
A digitized protocol that’s enriched with historical data and insights optimizes clinical trial execution for all stakeholders and systems by serving as the central, consistent, and accurate source of information. It facilitates the automated population of downstream systems, including electronic data capture (EDC), clinical trial management systems (CTMS), risk assessment platforms, and integrated quality management plans. This 'write once, read many times' methodology cuts down on manual data entry, saving countless hours during study setup, reducing human error, streamlining protocol amendments, and enhancing stakeholder collaboration.
Smart Risk Management: Integrating Critical Thinking with Digital Tools
While technological advancements are transforming risk management, human judgment remains the cornerstone of practical risk assessment. Our analytical skills and understanding of context are irreplaceable. Automation and data enrichment are potent tools to support this critical thinking—not supplant it.
A digital protocol plays a pivotal role in streamlining operations. It automatically identifies critical-to-quality (CTQ) factors and seamlessly populates data across various workflows. This includes everything from electronic data capture design and data/monitoring plans to configuring risk monitoring tools.
This integration delivers immediate benefits. Real-time notifications provide instant insight into changes in a study's risk profile, empowering teams to stay agile and proactively adapt their monitoring strategies. This is all achievable without the constant need to log into a centralized monitoring tool, significantly improving efficiency.
Furthermore, historical data is invaluable. It serves as a crucial reference, offering insights into previous risks and the successful mitigation strategies for specific study types and indications. This knowledge lets teams learn from the past and apply proven solutions to current and future challenges.
Elevating Site Selection: A Data-Driven Approach for Optimal Performance
Achieving optimal site selection in clinical trials demands a shift towards more robust, data-driven insights. This necessitates several critical considerations.
- First, a consolidated repository of historical site performance data is paramount. This includes key metrics like past enrollment rates, data quality metrics, incidence of protocol deviations, study timelines, regulatory compliance history, investigator experience, and site staff turnover. This holistic view provides a foundational understanding of a site's capabilities.
- Building on this foundation, developing and applying advanced algorithms are crucial for predicting future site performance. These algorithms should leverage historical data to forecast site enrollment capabilities and data quality. Incorporating factors like therapeutic area, geographical location, specific patient demographics, and detailed investigator profiles will significantly enhance their predictive power.
- A particular area where the industry has historically struggled is the creation of a genuinely comprehensive site/investigator performance score. While enrollment is often a primary focus, we advocate for dynamic, data-driven scores that provide a more balanced view. These scores should reflect a site's and individual investigator's track record in patient delivery and their consistent ability to generate high-quality data on time across specific therapeutic areas, patient populations, and study phases. This moves beyond basic investigator experience to quantify their proven ability to deliver quantity and quality.
- Next, patient population mapping is indispensable. By integrating real-world data (RWD) like de-identified and ethically sourced electronic health records, insurance claims data, and public health statistics, we can map disease prevalence and patient demographics to a granular geographic level.
Lastly, a comprehensive competitive landscape analysis must be integral to any robust site selection framework. Understanding the ongoing and planned clinical trial activity within a specific therapeutic area and geography is vital for assessing the competitive environment for patient recruitment at potential sites, allowing for more strategic and realistic enrollment planning.
Integrated Quality & Risk Management: The Digital Cockpit for Clinical Trials
The integrated quality and risk management plan (IQMP) is an essential advancement in clinical trial management. This isn't just a document; it's a dynamic framework that can be auto-created and driven by your digitalized protocol and robust risk assessment.
The IQMP becomes the central hub where all stakeholders can collaborate on a unified plan, eliminating the inefficiencies of siloed approaches. It provides clear visibility into who's doing what, allowing you to eliminate overlaps and close critical gaps.
Functioning as the cockpit orchestrating all downstream activities, a digital IQMP enables precise control over trial operations. It facilitates the setup of tools for data, site, and risk monitoring, ensuring real-time compliance checks. This intelligent system automatically highlights areas where too much or not enough emphasis is being placed, or where committed frequencies or scopes—like data review or site touchpoints—aren't being followed.
Real-time Risk Surveillance: Revolutionizing Central Monitoring with AI
Automated signal detection and analyses provide a holistic, real-time surveillance system for data quality, patient safety, and site performance. This means central monitors no longer need to spend hours sifting through various tools to identify individual signals and issues, such as those within key risk indicators (KRIs), quality tolerance limits (QTLs), or centralized statistical analyses.
The central monitoring universe can be enriched with metadata, where generative AI (GenAI) plays a vital role. GenAI can process audit data in real-time to pinpoint variability in site compliance, ensure proper data access and oversight, and even identify potential fraud and misconduct. These central monitoring-driven workflows seamlessly inform on-site monitoring activities through connected issue management, creating a truly integrated and proactive oversight system.
Dynamic Monitoring: Optimizing On-site Presence with Centralized Intelligence
Insights from central monitoring are revolutionizing on-site monitoring, significantly reducing the need for extensive on-site time and making traditional source data verification (SDV) less necessary. Integrating electronic health records directly with electronic data capture further diminishes monitoring activities focused on SDV; with no transcription, there are no transcription errors, effectively rendering SDV obsolete. This shifts the focus to source data review (SDR) and crucial process improvement conversations.
Any risks identified by on-site monitors are automatically fed back into central monitoring via integrated issue management. This ensures a continuous and efficient feedback loop with the overall risk management process, creating a dynamic and adaptive monitoring strategy.
Data-driven Excellence: The Foundation of 360° Monitoring
Insights and historical data can guide current and future studies, informing protocol design, risk assessment, and monitoring strategies. A comprehensive data collection lets teams adapt as needed, optimizing future protocols and feeding back into the 360-degree monitoring process.
Unified platform solutions are key for facilitating the 360° monitoring framework. They provide a centralized system integrating disparate data sources for enhanced oversight and streamlined processes. These technologies support seamless data integration and collaboration, enabling real-time analytics and effective RBQM across the clinical trial lifecycle.
Conclusion
The 360° monitoring framework offers a transformative approach to clinical trial oversight, integrating human critical thinking with advanced digital tools. It hinges on a digitized protocol as the central, accurate source of information, automating workflows from EDC design to risk assessment, significantly reducing manual effort and errors. This enables smart risk management with real-time insights and leverages historical data for proactive decision-making.
This framework also revolutionizes site selection through data-driven insights, including comprehensive site performance metrics and real-world patient population mapping. It provides an integrated quality and risk management Plan (IQMP) as a "digital cockpit" for unified stakeholder collaboration. The framework also deploys real-time risk surveillance powered by AI for continuous monitoring. In contrast, dynamic monitoring optimizes on-site activities by shifting focus from traditional SDV to data review and process improvement, ensuring a constant feedback loop throughout the trial lifecycle.
Learn more about how Medidata can help. Visit Medidata CTMS and Medidata Clinical Data Studio.
Contact Us
