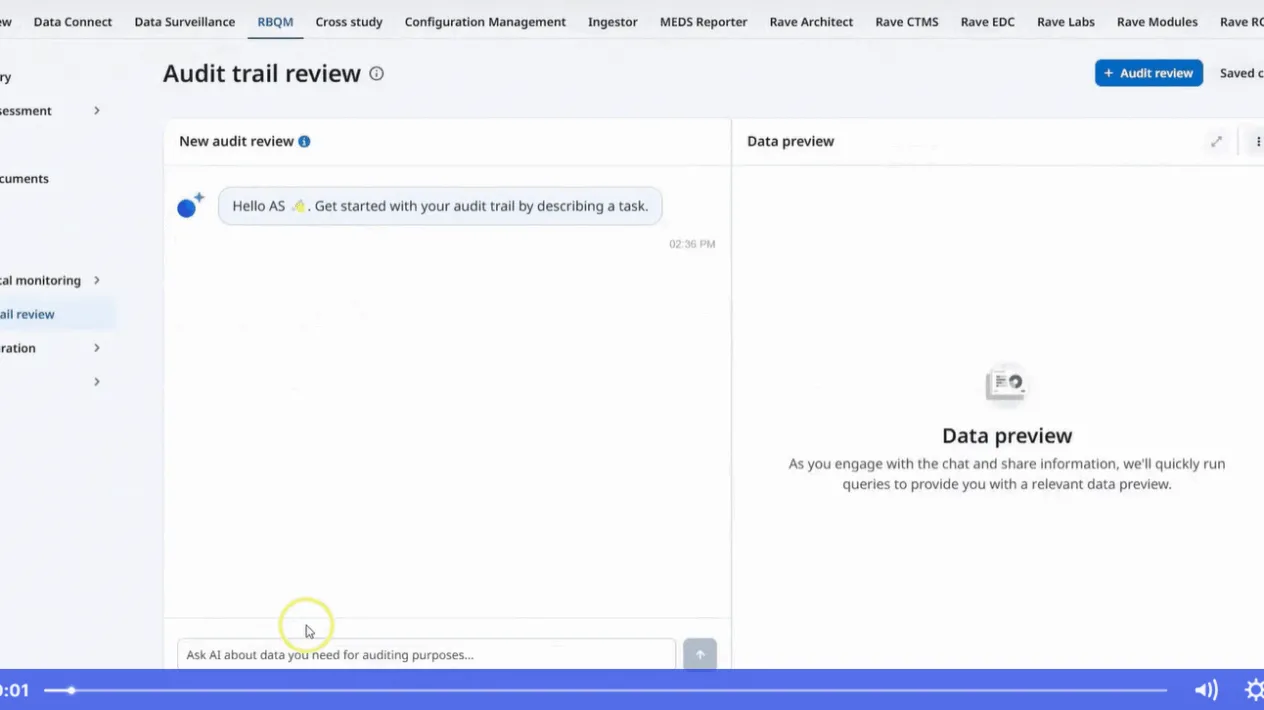
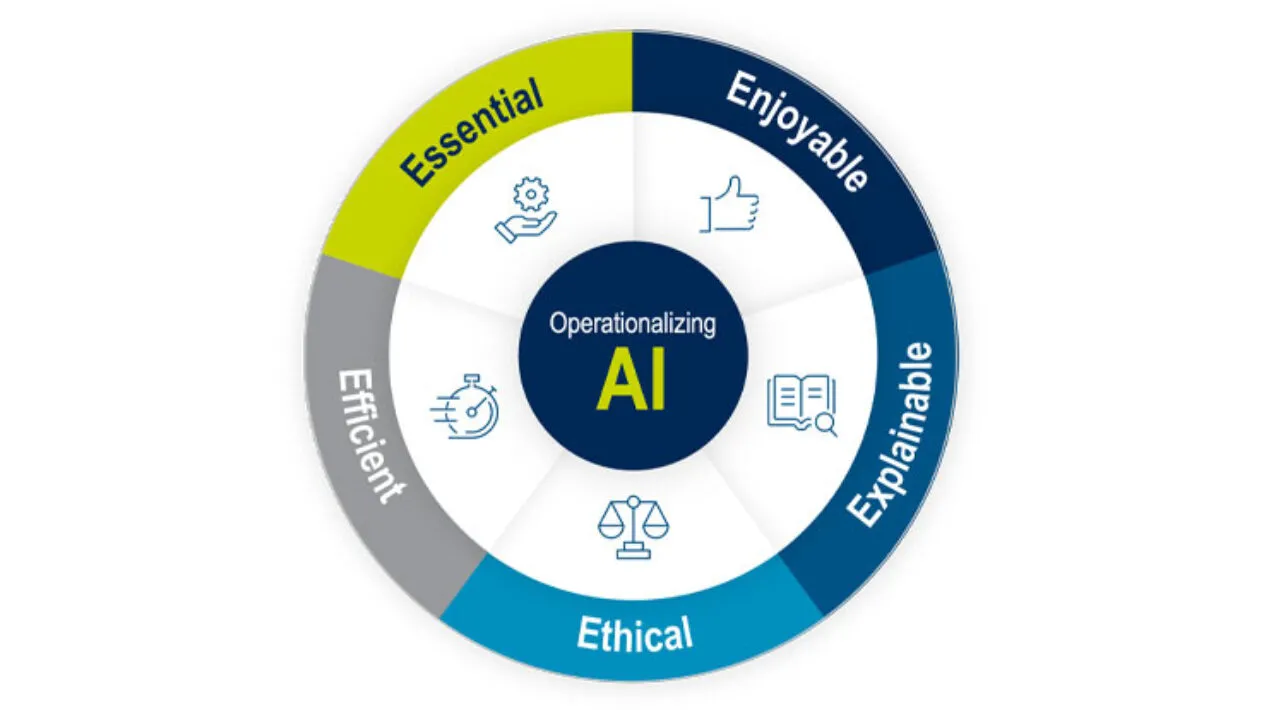
Empowering Transformation Through Technology From Clinical Data Management To Clinical Data Science
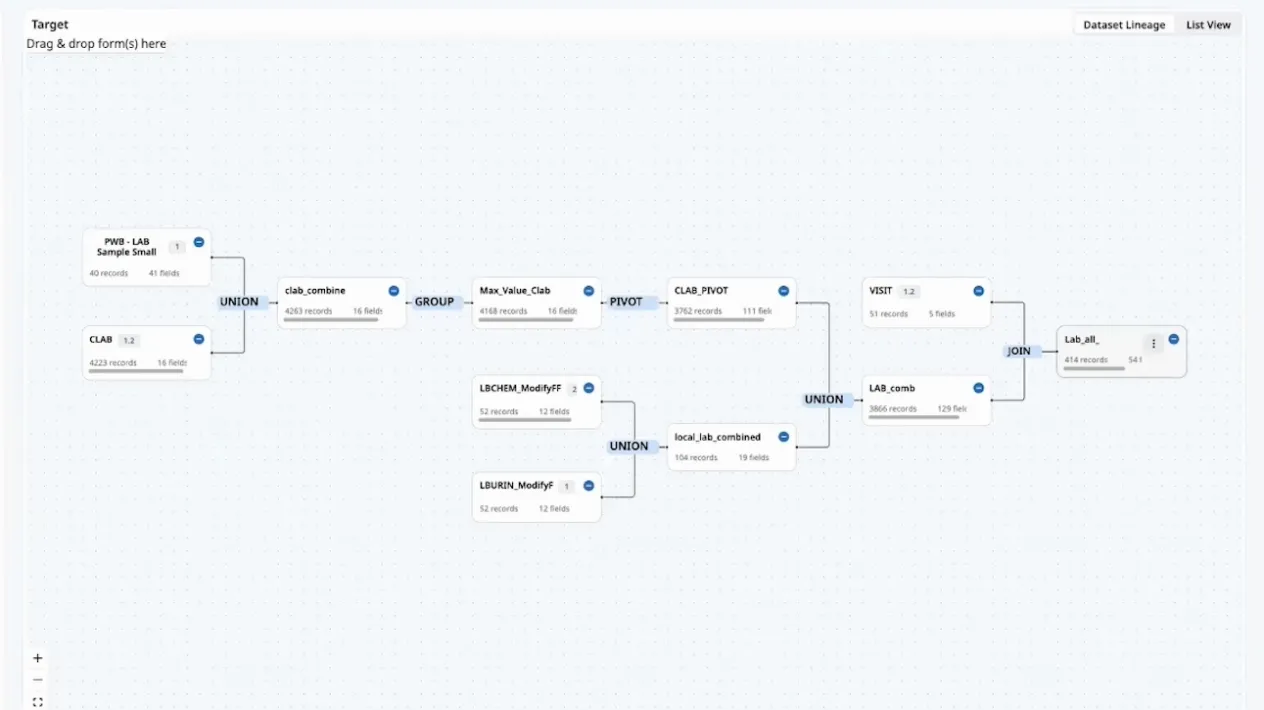
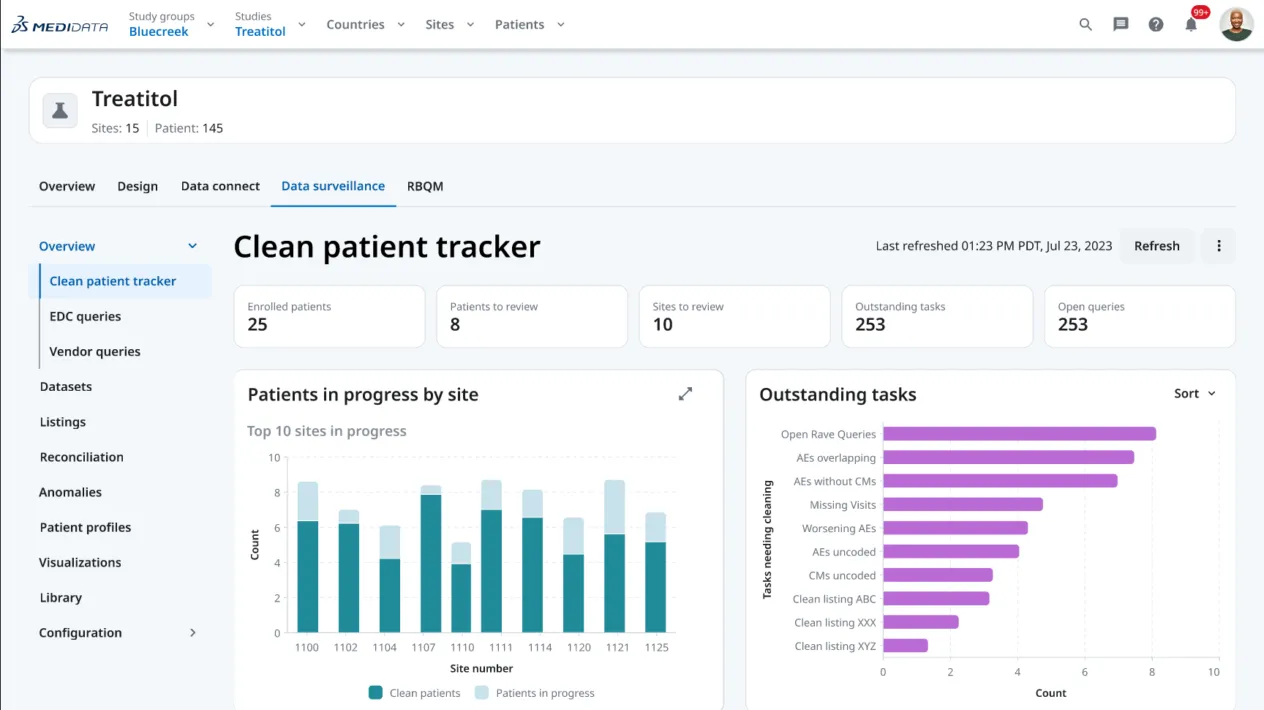
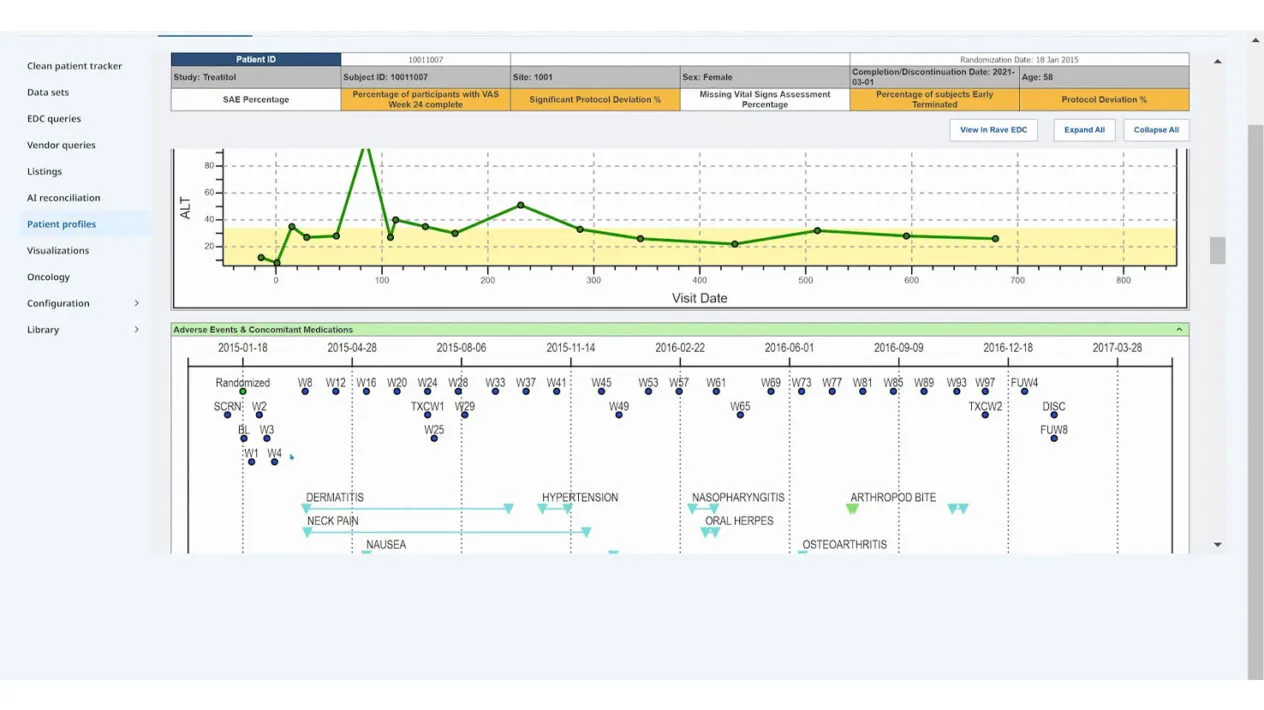
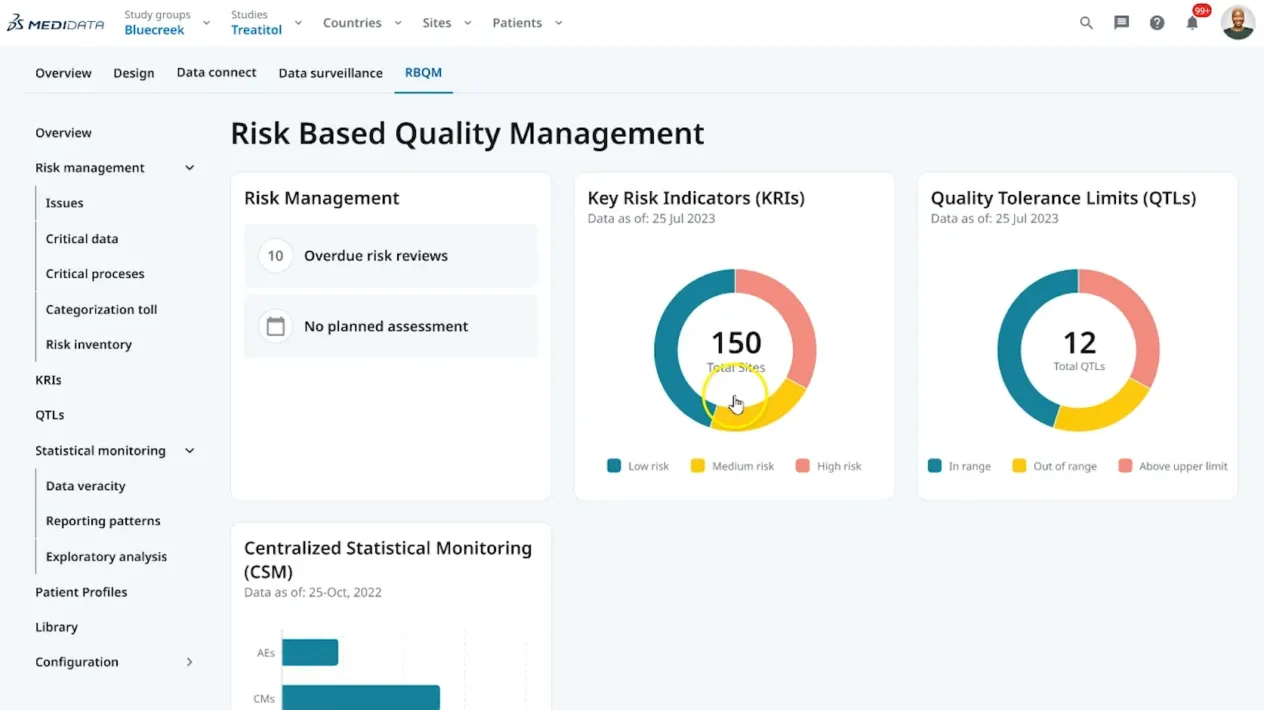
This article outlines the evolution of clinical data management into clinical data science, with data managers now being the stewards of all data and overall data quality. It highlights the critical tenets of innovative strategies and cutting-edge technology while ensuring a proactive, efficient, and insightful data management ecosystem.