Follow the Money – Innovations in End-to-end Clinical Trial Financial Management

Unsurprisingly, clinical trial financial management (CTFM) is complex. Surprisingly, a proper end-to-end CTFM solution does not exist… yet.
At Medidata NEXT New York 2023, Meghan Harrington, VP, Clinical Trial Financial Management, outlined why an actual end-to-end financial process is needed and shared an exciting announcement.
CTFM Complexity in Context
While the focus of any sponsor will be on meeting their clinical milestones, as Meghan stated:
“Financial management is the engine that drives clinical research activity.”
A study’s financial model mirrors a study’s operational complexity. Costs are compartmentalized according to operating models—usually employing disparate systems, services, and solutions. That complexity is exacerbated by using a mix of manual processes and workarounds, specialized clinical trial financial management software, and services, generating unique financial data sets within their functional remits. Critical areas impacted include site payments, patient payments and reimbursements, clinical trial supply management, vendor management, and overall study budget management.
In a Medidata survey, a sponsor shared the following:
“Manual processes and workarounds consume 80% of sponsors’ time and effort in their financial planning.”
This comment should be shocking, but it’s widely accepted as ‘normal.’ Sponsors apply a disparate mixture of solutions to address challenges—the equivalent of one hundred band-aids for one hundred problems.
Without an end-to-end CTFM platform, this practice of using disparate systems and processes (the band-aids) has its positives and negatives.
On the positive side, many independent specialized solutions are available to address specific problems within each operational layer. But implementing these disparate systems creates challenges—interoperability, integration with multiple systems, gaps or duplication of data, gaps in features, multiple user interfaces and logins, scalability, long-term software support issues (for heavily customized systems), and variable quality and availability of training (especially at a global level).
Disjointed processes lead to significant direct and indirect financial impact, so what can be done about this?
The Future of End-to-End Financial Management Is within Reach
Meghan announced that incredible innovations are on the horizon, as the Medidata platform is expanding to deliver a single, harmonized, end-to-end financial management experience for clinical trials.
Medidata Rave EDC is already an industry-standard solution integrated with Medidata’s suite of financial offerings. By adding patient payments and reimbursements in the second half of this year and further enhancements to its existing solutions, a sponsor’s financial team will be able to track every single dollar across the lifetime of a study—from early budget planning before draft protocols, all the way to a study close-out—an actual end-to-end financial experience (Figure 1).
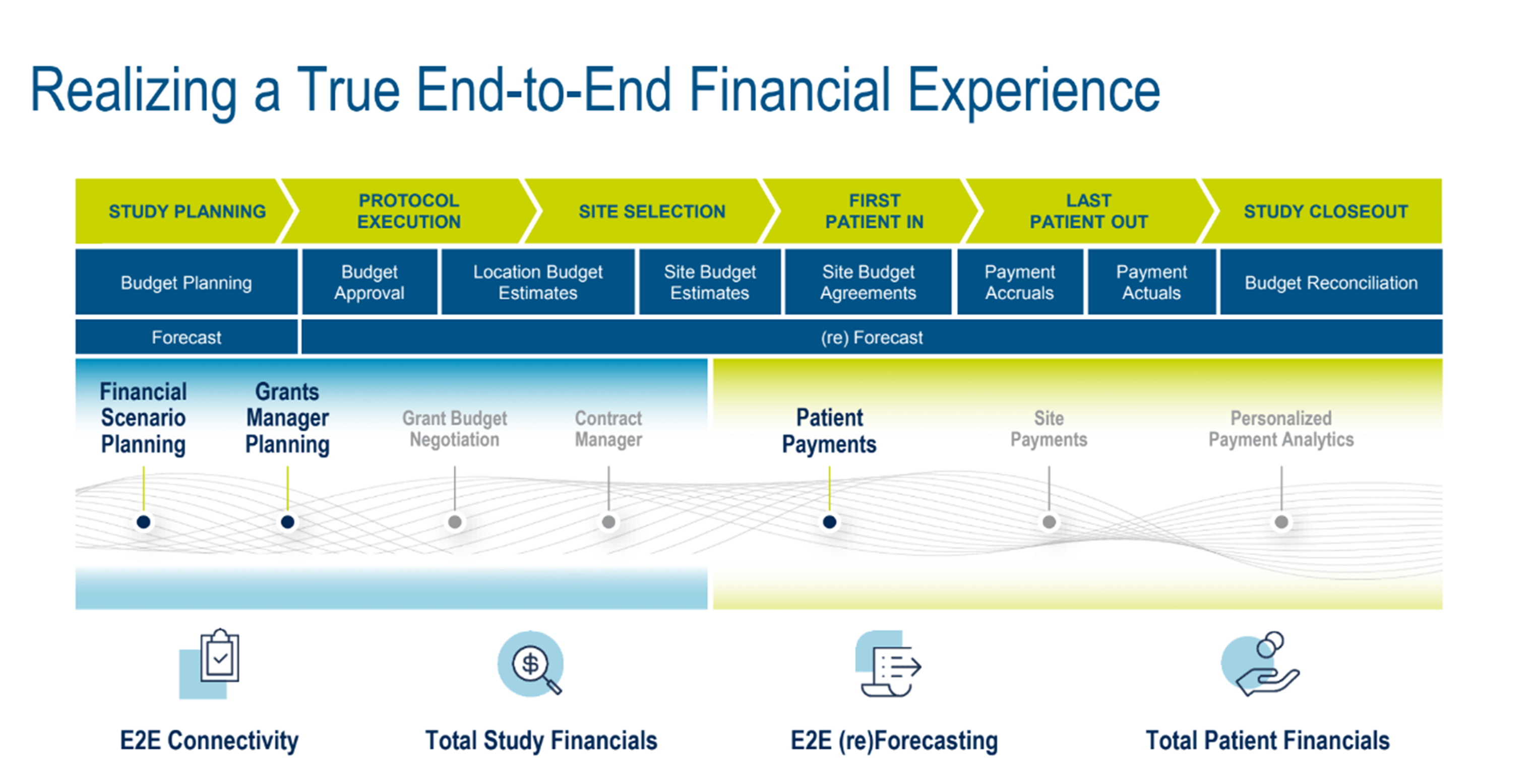
Figure 1. CTFM across the entire lifecycle of a clinical trial.
Meghan shared insights into three key innovations that make an end-to-end CTFM experience a reality.
At its core is data, the critical enabler on the path to innovation.
The site payments application has been used for almost $6 billion in grant payments in 52 unique currencies. It has generated benchmarking data that reflects 3.5 million itemized costs from over 450 sponsors and contributing partners in over 200 countries and regions.
This data has been leveraged to innovate across financial scenario planning, dynamic fair market value (FMV), and patient payments solutions.
Financial Scenario Planning
Clinical trial budget planning complexities are in a league of their own compared to other industries. In this dynamic environment, some essential information is often unavailable, so finance teams rely heavily on other data sources or their own experience, filling gaps with estimated data for many scenarios.
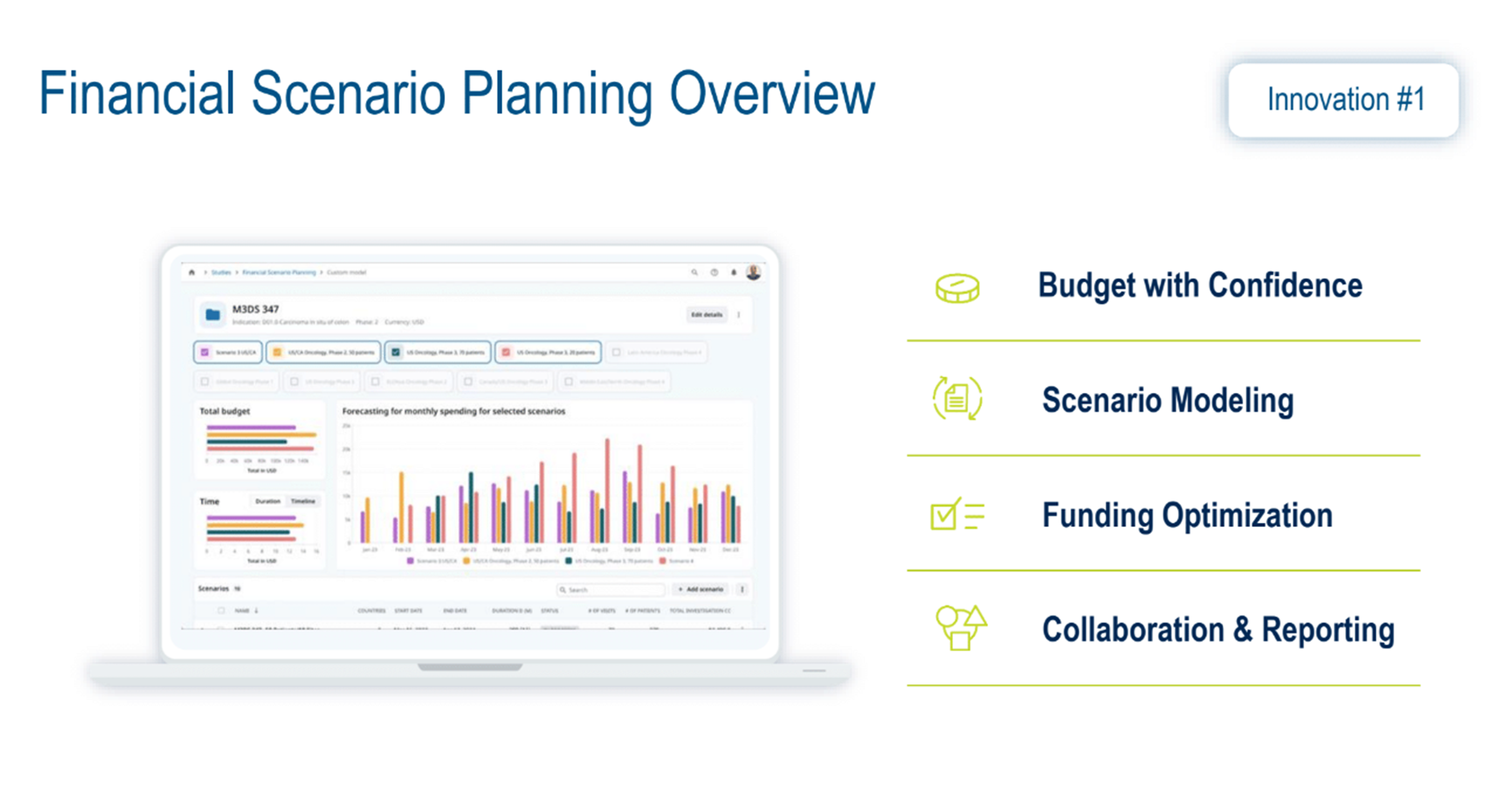
Figure 2. Financial scenario planning overview.
Medidata’s financial scenario planning solution (Figure 2) addresses the current gap in the industry by aligning early budget modeling with feasibility-aligned forecasting, leveraging vast volumes of benchmark data. Advanced analytics and statistical modeling empower users to build, assess, and optimize multiple scenario models, ensuring stakeholders can make decisions based on data-driven insights and forecasts.
Dynamic Fair Market Value (FMV)
Assessing what constitutes fair payments for sites within the clinical ecosystem is a crucial challenge. Industry averages are not enough. Variations globally, nationally, and locally need to be considered when creating defensible financial benchmarking for clinical studies.
Meghan shared insights into how Medidata applies innovative approaches to leveraging data, empowering users to make better decisions. For the FMV applied within Medidata Rave Grants Manager Planning, this included using committed data (extracted data from executed contracts), anticipated data (committed data and third-party data sources), country ratios, exchange rates, inflation & statistical models, and projected data (predicted data based on the length of the study) (Figure 3).
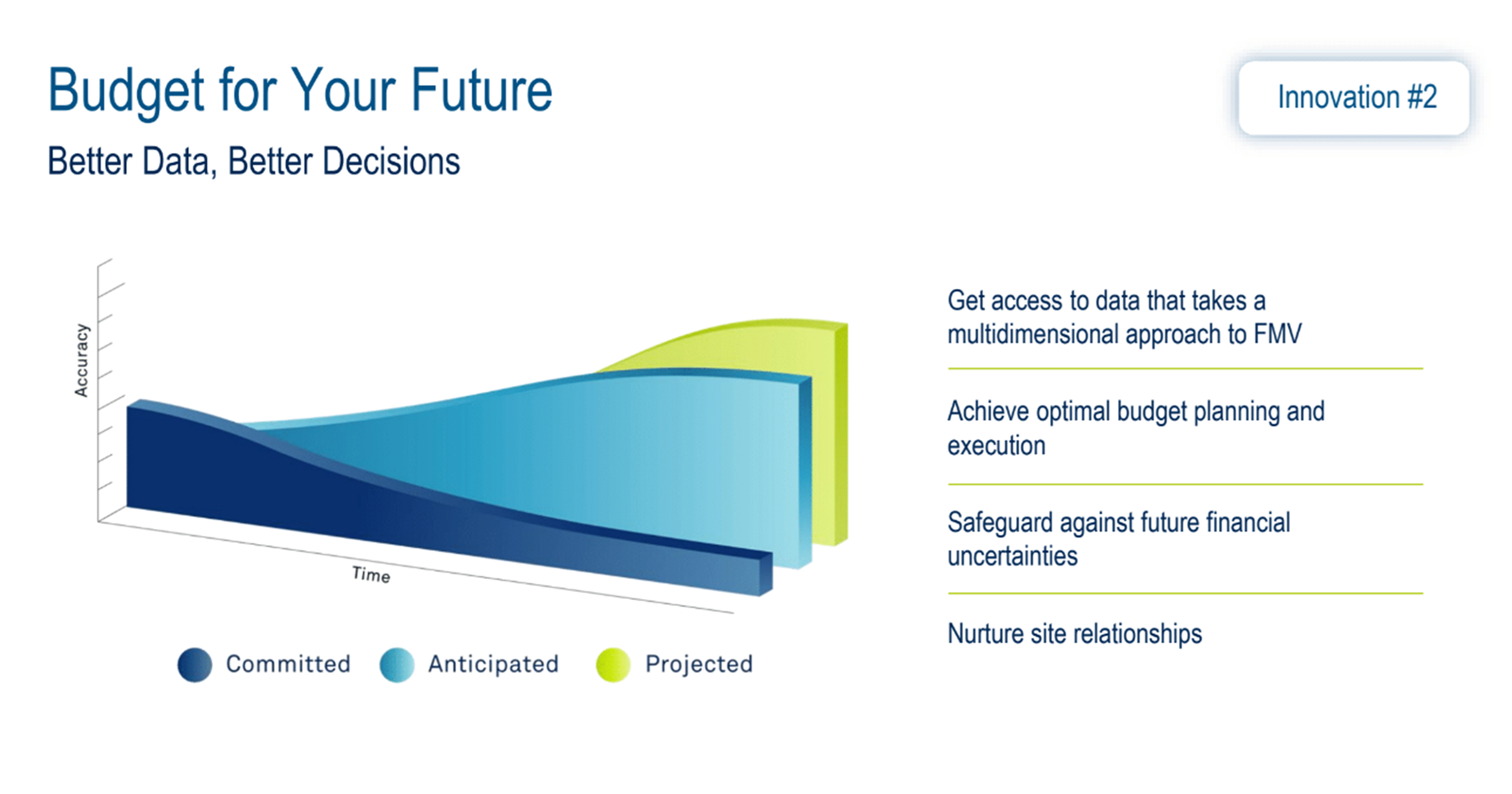
Figure 3. Fair market value.
AI is used to help conduct complex and deep analyses and projections, adapting to dynamic financial changes like inflation rates. Medidata’s Grants Manager is already recognized as an industry standard, so the innovations have enhanced this leading solution even further as part of the CTFM end-to-end offering.
Patient Payments
Patient payments/reimbursements will be introduced in the second half of 2024. This solution builds on the strengths of the myMedidata app and existing Medidata products (Figure 4). A system preview was shown, demonstrating that it’s been built with the patient experience in mind.
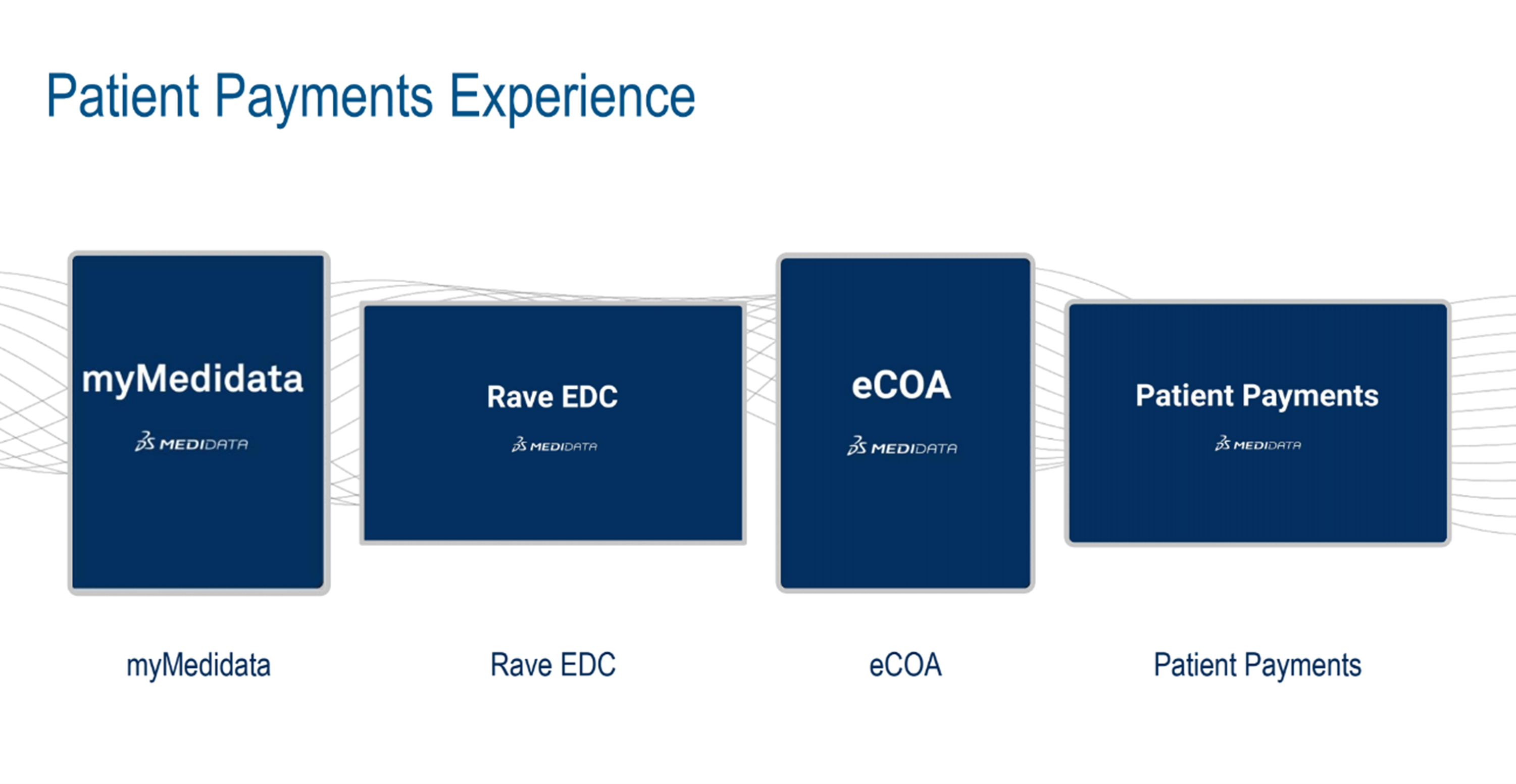
Figure 4. The patient payments experience.
Patient payment and reimbursement solutions reduce patient burdens and aid retention. By leveraging its entire solution—the most widely adopted clinical data platform—Medidata aims to remove any inconvenience from the process.
In Conclusion
The industry has tried its best to create harmonized financial and operational environments for decades by piecing together a jigsaw puzzle of disparate systems and processes. But sponsors have made it clear—a unified, harmonized end-to-end financial management platform is needed. And it’s finally on the horizon.
Watch the full presentation here to learn more.
To hear more about the innovative things the Medidata clinical trial financial management team is doing, visit the CTFM Resources page.
Explore Related Articles
Contact Us

