Medical Device Clinical Trials: What You Need to Know

What Are Medical Devices?
As defined in Section 201 of the Federal Food, Drug, and Cosmetic Act, a medical device is:
“An instrument…intended for use in the “diagnosis…cure, mitigation, treatment or prevention of disease…and primary purpose not through chemical action within or on the body…”
This differs from in vitro diagnostics (IVD) products, which are defined in Title 21 of the United States Food and Drug Administration (FDA)’s Code of Federal Regulations as:
“...reagents, instruments, and systems intended for use in diagnosis of disease or other conditions…in order to cure, mitigate, treat, or prevent disease or its sequelae. Such products are intended for use in the collection, preparation, and examination of specimens taken from the human body…”
What Are the Classifications of Medical Device Trials?
The FDA divides medical devices into three classes based on their risks and the regulatory controls needed to provide a reasonable assurance of safety and effectiveness. These classifications are slightly different from those found in Canada and the EU.
Class I
These devices pose a low level of risk. Examples include bandages, exam gloves, and handheld surgical instruments. Most Class I medical devices are exempt from review and only require registration before they can be marketed, while some devices require an additional 510(k) notification.
Class II
These devices pose a moderate level of risk. Examples include powered wheelchairs, infusion pumps, and surgical drapes. Most Class II medical devices must submit a 510(k) notification for review before being marketed.
Class III
These devices pose a high level of risk. Examples include heart valves, silicone breast implants, and implanted cerebella stimulators. All Class III medical devices must submit a premarket approval (PMA) application before being marketed.
Where Are New Medical Device Clinical Trials Emerging Globally?
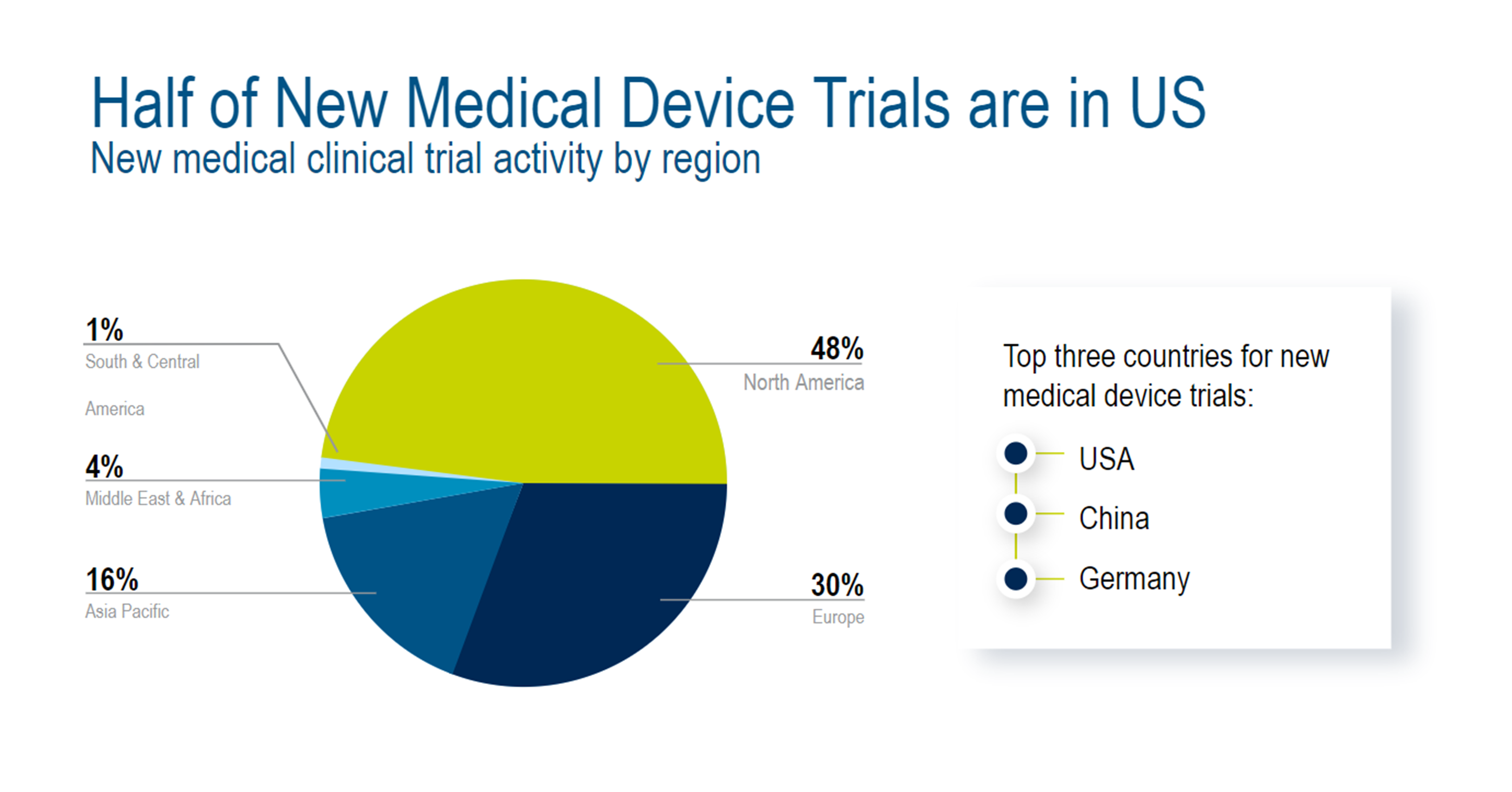
The global landscape for medical device clinical trials is primarily concentrated in North America, Europe, and Asia. According to 2022 research, North America had the largest share of new medical device trial activity (48%), followed by Europe (30%) and Asia-Pacific (16%). The top three countries for new medical device trials are the US, China, and Germany, respectively.1
Which Therapeutic Areas Make Up New Medical Device Trials?
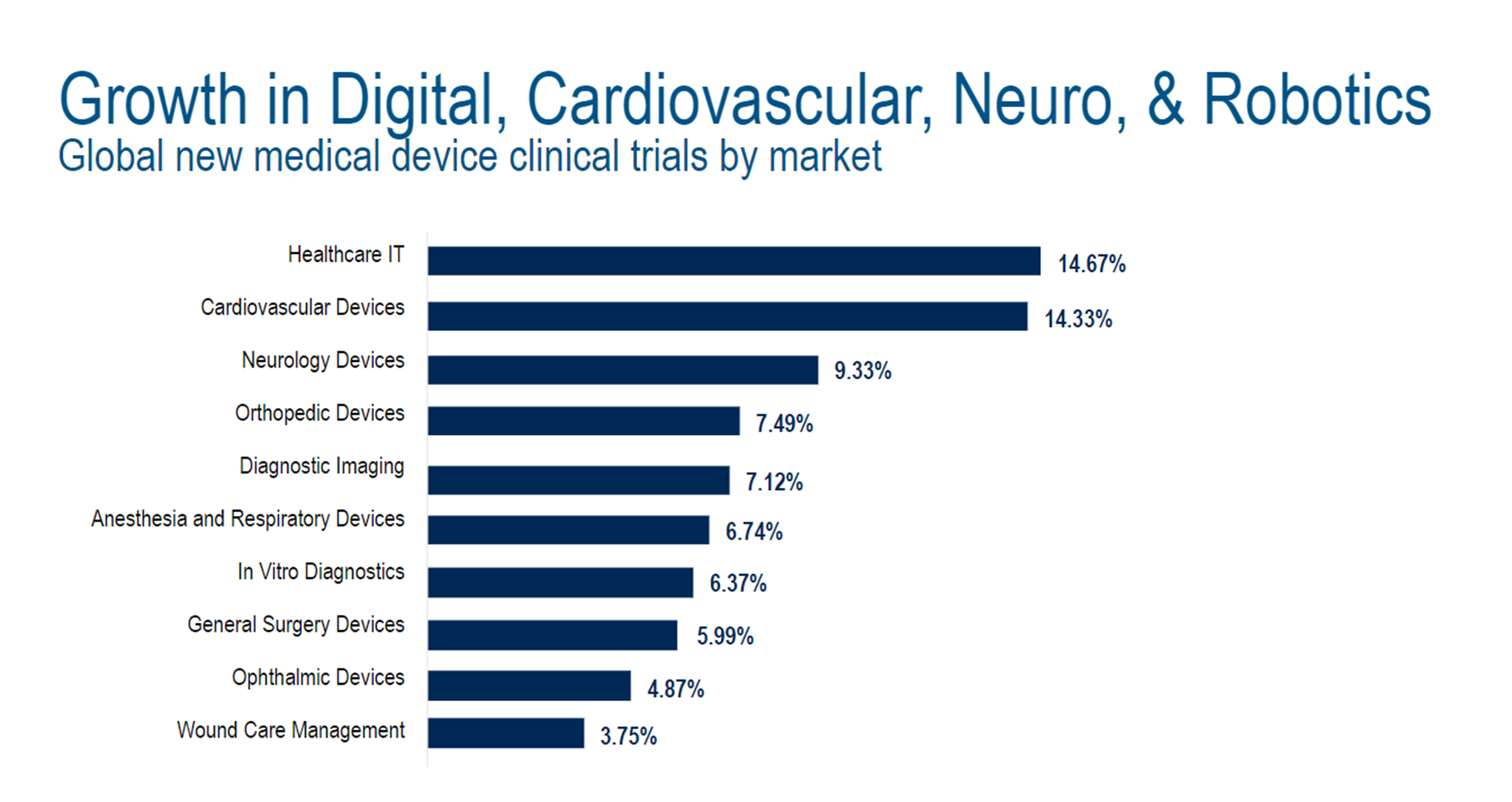
Healthcare IT was the leader in new medical device clinical trials by market (14.7%), followed by cardiovascular devices (14.3%), neurology devices (9.3%), orthopedic devices (7.5%), diagnostic imaging (7.1%), and anesthesia and respiratory devices (6.7%).2
What Are the Unique Challenges that Medical Device Clinical Trials Face?
Medical device trials have specific pain points spanning study start-up, enrollment, closeout, and commercialization.
Patient Enrollment – Especially difficult for medical device trials, as patient recruitment requires extensive assessment and support.
Regulatory Complexity – Complicated by different regulatory bodies across the globe. Data privacy regulations and outdated documentation systems are also significant pain points.
Economic Value – Demonstrating value is a must; incorporating economic endpoints into clinical study protocols can dramatically improve commercial success.
What Are the Differences Between Medical Device Trials & Drug Trials?
Medical device trials and drug trials differ in terminology, study design, study timelines, and data needs.
First, medical devices have their own stringent set of requirements to demonstrate safety and effectiveness in a clinical setting. These trials follow a different path to approval compared to pharmaceuticals. While drug trials follow phases (I-IV), medical device trials have unique stages and product classifications.
Unlike drug trials, placebos are rare for medical device trials. Blinding is also rare and difficult, while randomization and crossover studies are less common.
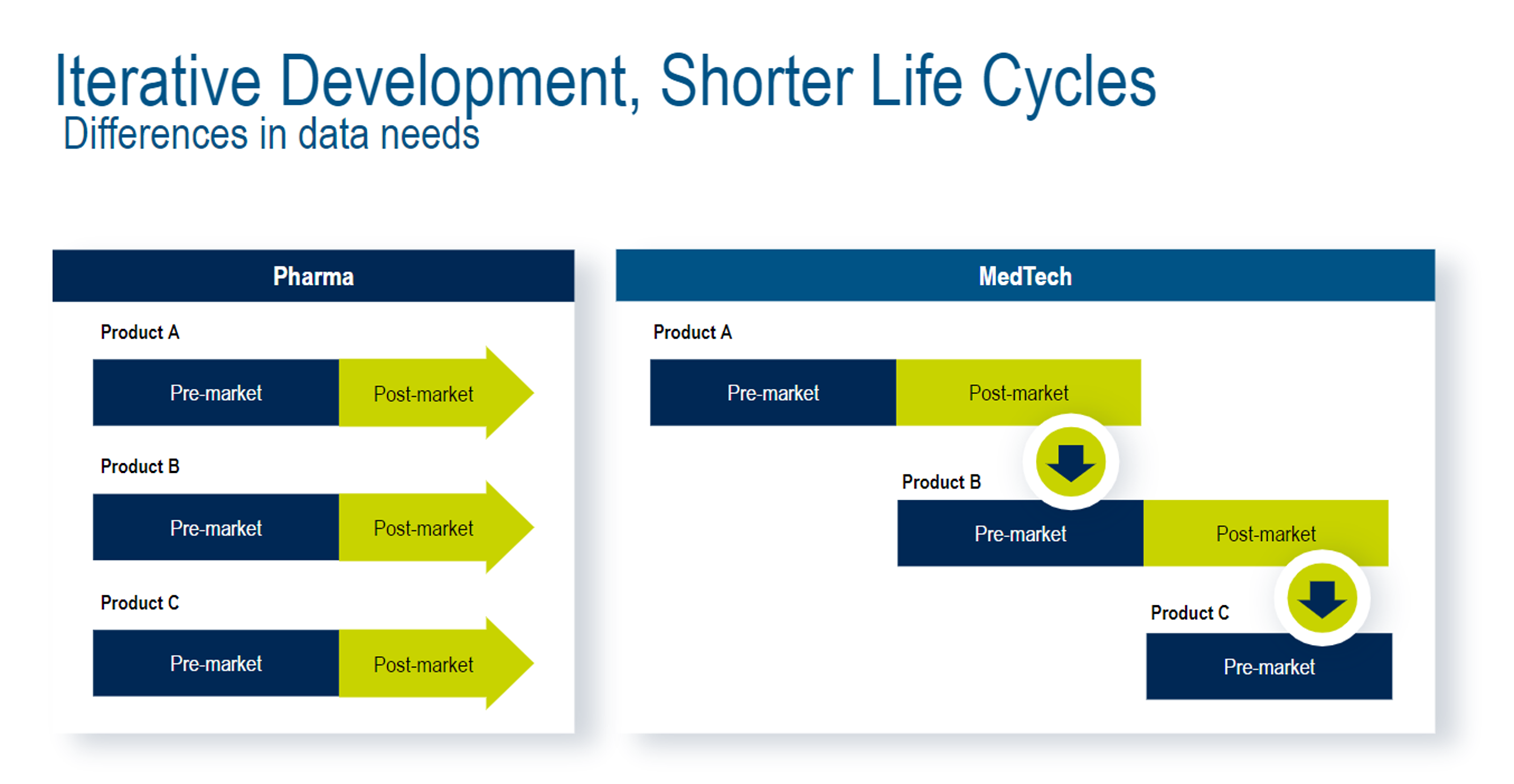
Drug trials and medical device trials also have different data needs for product innovation. In drug trials, companies will get approval for a specific therapeutic and then begin work on a different therapeutic. On the other hand, medical device trials are more likely to follow an iterative process. The insights gathered in post-market data collection are often fed back into early product development stages to make slight modifications.
What Are the Stages of Medical Device Clinical Trials?
There are four clinical trial stages for medical devices, ranging from small studies to long-term monitoring.
Pilot / Early Feasibility / First-in-Human
This initial stage is a small trial (between 10-30 patients) meant to collect preliminary safety and device performance data. Findings from this study guide device modifications and future study design.
Traditional Feasibility
This stage is a small study (between 20-30 patients) that assesses safety and efficacy of the near-final or final device design in patients. Traditional feasibility studies inform the design of the pivotal study.
Pivotal
The pivotal stage is a large trial (hundreds of patients) that confirms clinical efficacy, safety, and risks associated with the device. This stage is statistically driven.
Post Market
This stage (thousands of patients) monitors long-term effectiveness, safety, and usage in the general population after the device has gone to market.
What Does the Regulatory Landscape Look Like for Medical Device Clinical Trials?
The United States (US), European Union (EU), and United Kingdom (UK) have different regulatory bodies and requirements to run medical device trials. Below is an overall look at the landscape, as well as the common regulatory themes across the industry.
US Regulations
The FDA regulates medical devices in the United States. Manufacturers and distributors must register with the FDA to introduce devices to market. The FDA has continued to modernize its processes and requirements to keep pace with the rate of scientific change, while also increasing scrutiny of data integrity.
EU Regulations
European Union regulations have changed to take a total life cycle approach to monitoring medical devices. These parameters (known as EU Medical Device Regulation, or EU MDR) aim to improve the industry’s transparency, safety, and efficacy.
Over the coming years, companies will be required to complete reapprovals of past devices and collect more real-world safety and efficacy data. Manufacturers must prove clinical safety and performance of their devices throughout their expected lifetimes. There is also new oversight being introduced as part of EU MDR, including a risk-based classification system and the introduction of an expert panel to act as impartial performance evaluators and risk reviewers.
UK Regulations
The United Kingdom has its own authority responsible for medical device regulation: the Medical and Healthcare Regulatory Agency (MHRA). The MHRA oversees market surveillance, supply and marketing decisions, and the designation and monitoring of UK assessment bodies. All medical devices require MHRA registration to enter the UK market.
Common Regulatory Themes
Below are some common themes across medical device regulation:
- Patient safety and centrality
- Data standardization
- Data quality and data integrity
- System integrity and validation
- Improved traceability
- Improved transparency
- Generating greater amounts of clinical evidence data
- Ability to aggregate and submit clinical evidence data
- Integrating and aligning multiple data sets for regulatory submissions
Learn more about regulatory requirements for medical devices.
What Do Medical Device Companies Need from Clinical Trial Solutions?
Medical device companies need a unified, comprehensive clinical trial offering for the entire product life cycle.
A solution that connects people, processes, and data.
One that delivers a single source of truth to feed data insights from the post-approval space into product development.
And provides unmatched support and cross-industry expertise grounded in rigorous scientific standards.
All while keeping the patient at the heart of innovation and product development.
Get Started Today
Discover how Medidata helps MedTech leaders achieve clinical, regulatory, and long-term success.
References
1, 2. Medical Device Network, June 2022: https://www.medicaldevice-network.com/marketdata/new-medical-device-clinical-trials-june-2022/?cf-view
Contact Us
