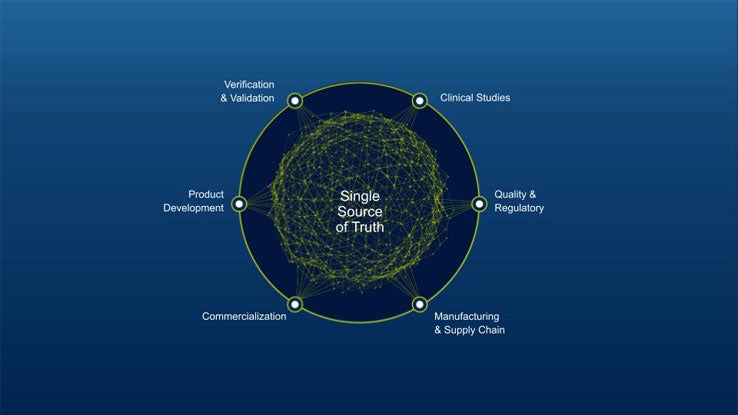
Your Connected MedTech Ecosystem:
Reimagine the Process of Innovation
Confidently navigate the MedTech value chain and expedite your products to market with interconnected intelligence and collaboration solutions. Because patient care can’t wait.

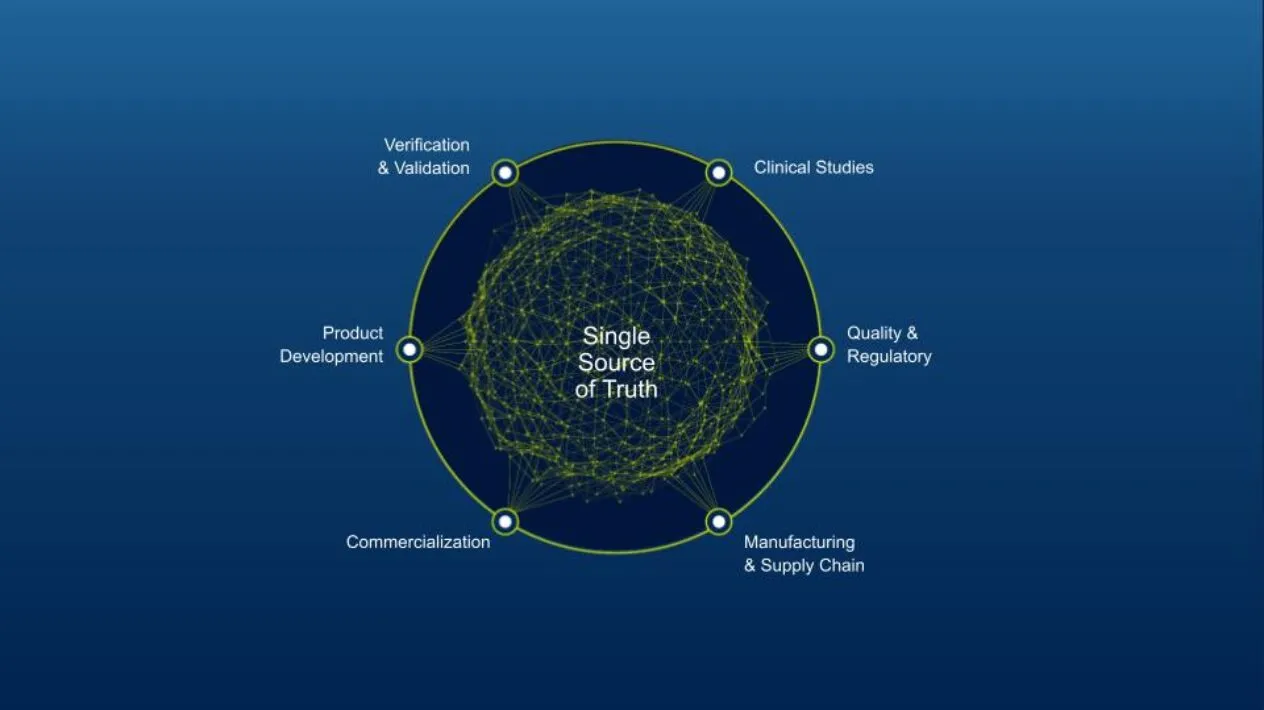
The MedTech Ecosystem
The MedTech ecosystem is a suite of digitally connected science and engineering applications designed to transform innovation through every medical device and diagnostics product lifecycle stage. Explore unlimited scenarios and predict future behaviors with Virtual Twin-powered possibilities and patient-centric, AI-powered insights.
Product Lifecycle Solutions For Personalized Medicine
Product Development
Visualize your medical device from every angle with linear and 3D modeling, and simulate real-life behavior to assess critical testing points. Design right the first time with product development solutions, including SOLIDWORKS, SIMULIA, CATIA, and more.
- Design next-gen biological, chemical, and materials at the atomic and molecular levels.
- Engineer your products using powerful 2D and 3D computer-aided design.
- Assess medical device performance and reliability with realistic 3D prototypes.
- Evaluate and predict your product’s behavior in multiple real-life scenarios through a model-based approach.
- Bring products to market faster using virtual twin technology as decision support and evidence for in silico clinical trials.
Verification & Validation
Ensure your medical devices meet design requirements and patient needs with validation and verification solutions.
- Accelerate the process of evaluating materials and product safety.
- Reduce the validation effort for testing medical device functionality.
- Verify your compliance with industrial regulations quickly.
- Facilitate cross-disciplinary collaboration and solve underlying design issues early.
Clinical Studies
Fast-track your medical devices with Medidata, the only eClinical solution that helps you run your trials, engage with patients, sites, and sponsors, and leverage the latest clinical research data.
- Smooth the patient recruitment and study experience before, during, and after clinical studies with unmatched patient centricity.
- Simplify and improve clinical data quality with a unified clinical data management solution.
- Design patient-centric studies with AI intelligence and synthetic control arms.
- Transform clinical operations workflows for a shorter study timeline.
Quality & Regulatory
Activate a Total Quality Management (TQM) program to comply with ever-changing regulations to get and maintain market approval, manage risk, and meet patient safety demands.
- Move beyond compliance to full traceability across a unified digital thread.
- Continually innovate across your value network in a Product Lifecycle Management (PLM) collaboration environment.
- Effectively capture customer product usage and risks for post-market surveillance.
- Confidently navigate international, regional, and national standards from IEC, ISO, and WHO to FDA, EU MDR, UKCA, and more.
Manufacturing & Supply Chain
Sustainably accelerate your operations to minimize deviations and waste with a virtual twin of your plant assets, supply chain, and production processes.
- Digitally model your ideal-state production process to create as-built products to match as-designed products.
- Virtually commission, validate and optimize production lines, workflows, and employee ergonomics.
- Achieve next-gen supply chain resiliency with a fully connected supply chain network.
- Proactively monitor plant equipment maintenance for maximum uptime.
Commercialization
Assess how your medical device performs in the real world and adapt your products as your patient experiences evolve.
- Market products using rich, interactive 3D content experiences.
- Personalize surgical planning with seamless collaboration between device manufacturers, surgeons, and other HCPs.
- Monitor post-market activities to track and reduce the number of adverse events and product recalls.
- Link real-world data (RWD) with clinical trial data (CTD) for effective safety tracking.
Why Choose Dassault Systèmes
What Our Customers Have to Say
Case Studies
Learn how airflow simulations and computational fluid dynamics software assisted Avidicare in reducing airborne contamination in sensitive environments. See SIMULIA in action.

Discover how the 3DEXPERIENCE platform and virtual modeling enables Biomodex in bringing realistic 3D-printed organs for physician procedure training quickly to the market.

Learn how Flow Robotics develops liquid handling robots using the 3DEXPERIENCE platform to leverage multidisciplinary collaboration, significantly improve workflow efficiency, and produce better designs with higher success rates.

Discover how Medtronic utilizes the 3DEXPERIENCE platform and realistic simulation to rapidly identify critical parameters of their stent graph device, significantly reducing design cycle time while increasing its reliability.

Explore how Mevion leverages Dassault Systèmes’ simulation tool to optimize their proton therapy systems for cancer patients.

Learn how Neusoft redesigned its entire production traceability and materials flow, saving time and costs with DELMIA.

Discover how WSAudiology developed leading-edge hearing aids with less product development cycles and maximum innovation fueled by SIMULIA simulation.

Additional Resources
Dive deeper into the medical device industry solution experience. Explore our latest resources.
View Case Study
Download eBook
Download eBook
Download eBook
Read White Paper
Download Guide
Download eBook
Interested in Learning About Your Innovation Readiness?
Let our industry, science and engineering experts assess your readiness:
- Increase Speed to Market
- Patient-centric Study Design
- Simplify Regulatory Compliance
- Operate Sustainability
- Post-market Surveillance