Rave RTSM
Rave RTSM is the only fully pre-validated randomization and trial supply management solution that can be configured in minutes and enables mid-study changes with minimal downtime and change orders.
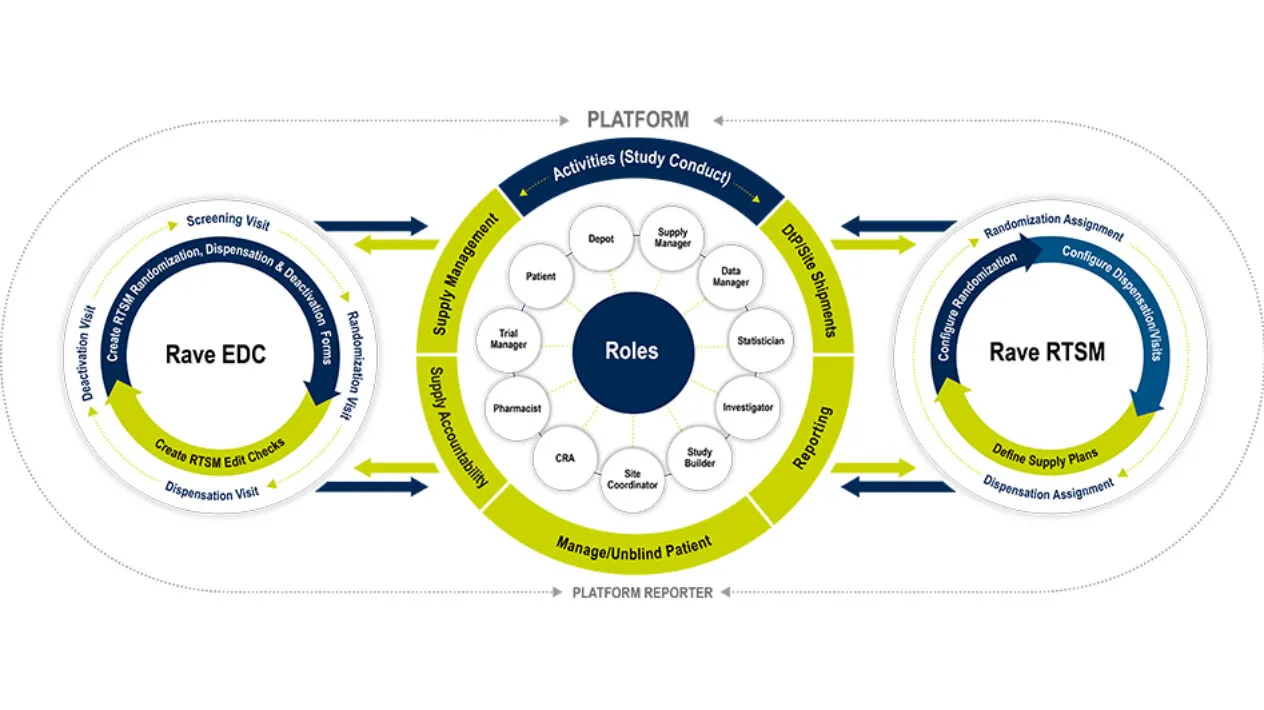
RTSM is built on Rave EDC, so there is no double data entry and minimal reconciliation expediting study start-up and study-close out. Rave RTSM streamlines your operations and provides real-time visibility for your study teams.
Key Capabilities
Leading the competition with next-generation reporting and analytics
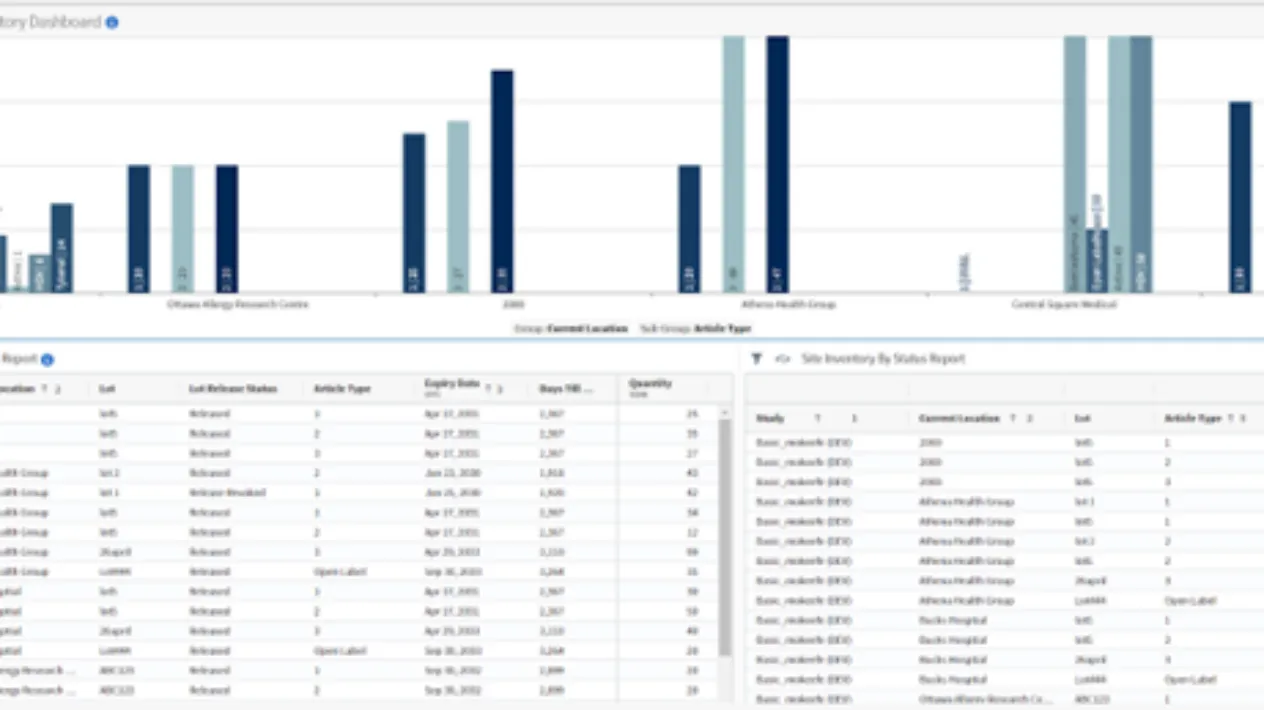
Real-time visibility into enrollment, randomization and supply enabling real-time decision making on needs to increase/decrease supply at site while minimizing waste. Custom reports and dashboards enable visualization of data through charts, graphs, plots and maps.
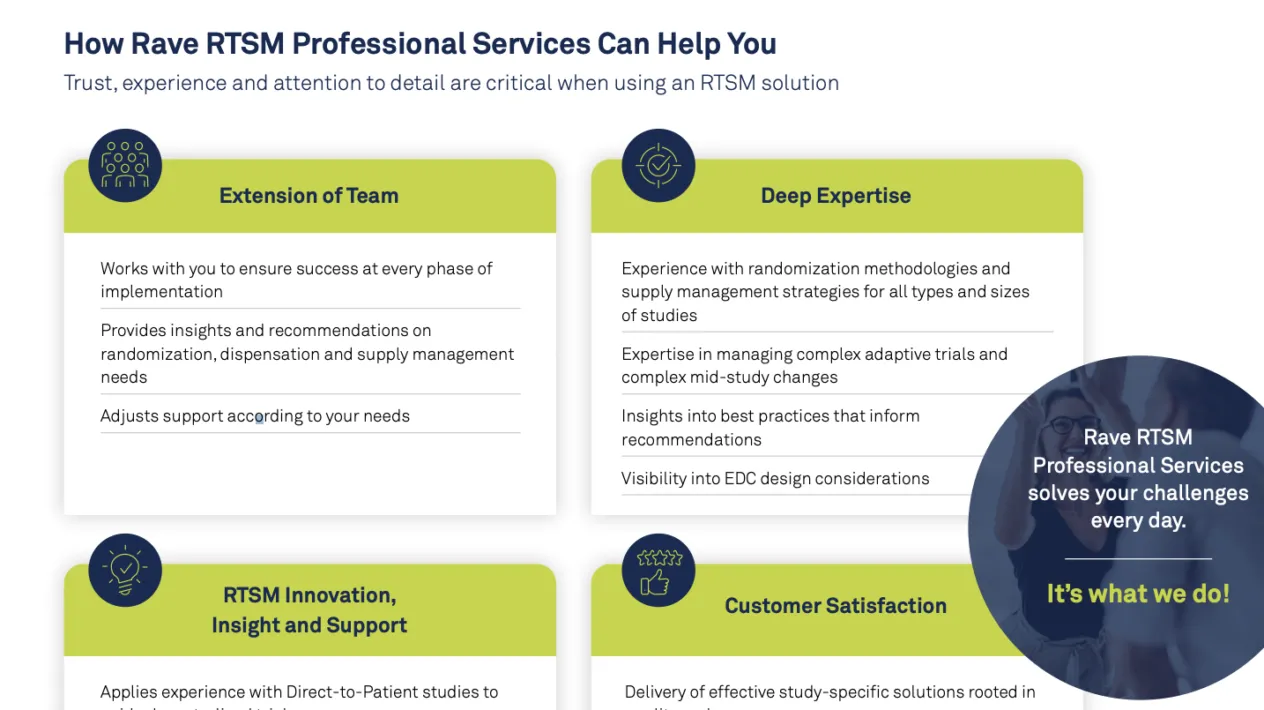
Unparalleled Experience and Expertise in RTSM
Medidata’s Professional Services RTSM team of experts has countless years of clinical trial experience and a deep understanding of the IRT / RTSM industry. We partner with you every step of the way to ensure your RTSM processes are optimized so you can achieve the highest value. Support can range from full build and execution to Live Study Management™, which allows you to have an experienced IRT SME who can support unblinded trial supply management activities during study conduct phase. Among the benefits include guiding you towards the most impactful supply strategy to meet your goals.
Robust Supply Management Tools
Rave RTSM’s supply management capabilities and real-time visibility provide the data and analysis to ensure supply optimization. RTSM’s supply plans offer the height of flexibility to make real-time decisions on supply planning. RTSM integrates with the industry’s largest depots, which ensures expedited shipping. Cross-study reporting, drug pooling and forecasting are just some examples of how to accomplish your supply chain objectives.
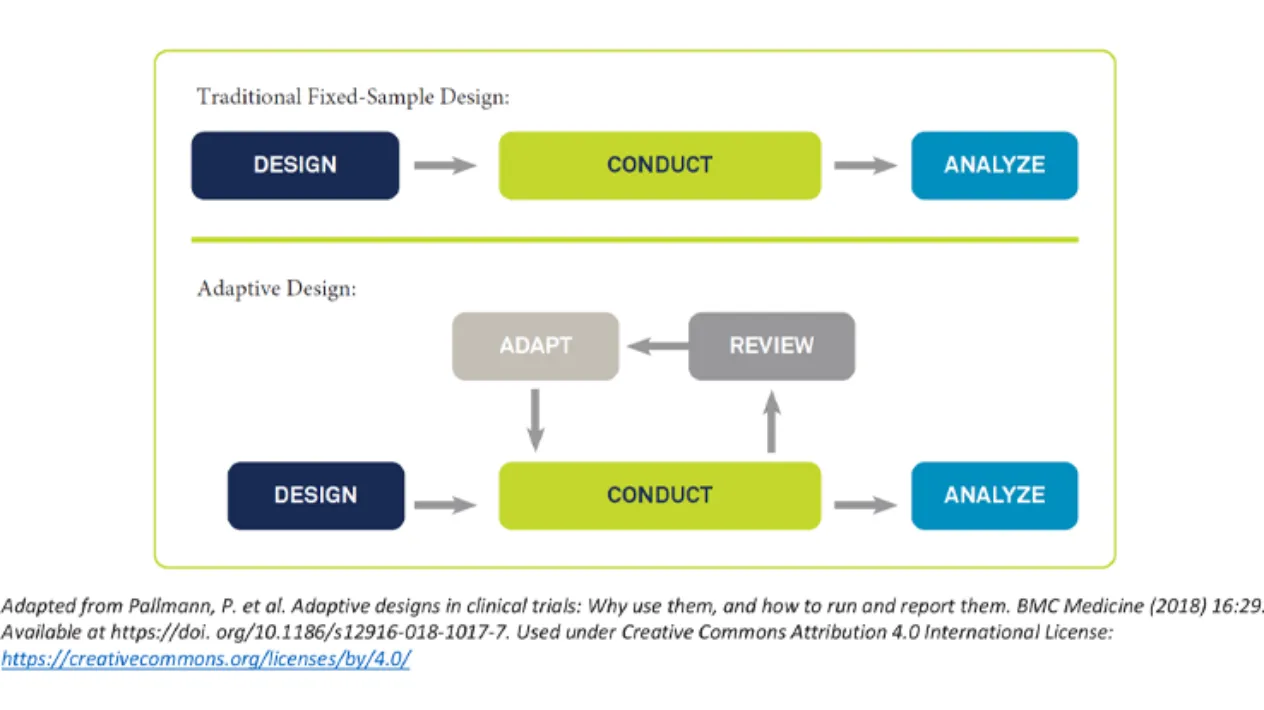
Build and Execute Complex Adaptive Trials Quickly and Efficiently
Rave RTSM has the flexibility to quickly and seamlessly adapt to changes in clinical trial designs using pre-validated randomization and trial supply solutions that can be configured in minutes. Complex adaptive, cohort, umbrella and basket trials are easier to build while mid-study changes can be made in minutes with no down time or change orders. Expedite study adaptations quicker across products on the Medidata Platform.
Ease and Flexibility to Execute Direct-to-Patient (DtP) in Real-time
Rave RTSM has unsurpassed DtP capabilities giving sponsors and CROs the highest level of flexibility for decentralized and hybrid trials. DtP can be enabled for any combination of sites and visits and provides sites the ability to determine the source of the patient’s dispensation.
In addition, only the Medidata Platform is truly virtualized allowing patients to confirm incoming shipments using the myMedidata App.
What Customers Have to Say
“After 30 years in Pharmaceutical Supply Chain, I have found Medidata Rave RTSM to be the most complete and user-friendly IRT system to support clinical supplies.”
– Todd Zegarzewski, Sr. Supply Chain Manager
“Rave RTSM and the RTSM PS team complemented each other perfectly in our study. Especially in complex studies with different study materials, Rave RTSM is extremely helpful in maintaining an overview at all times. We are able to control the flow of study materials and make adjustments to the inventory on our own in Rave RTSM. Medidata’s teams were always ready to help with any questions and could always rely on the competent and quick help of our contact persons. All in all, we were absolutely satisfied with the tools and services offered!”
– Dr Bernd Ullrich, Senior Clinical Trial Manager, Heidelberg ImmunoTherapeutics
“Medidata has made a tremendous impact on how MyMD Pharmaceuticals has been able to conduct its clinical research. Their comprehensive suite of services has simplified aspects of the clinical trial process, from randomization [with RTSM] to data collection and analysis. Through the platform’s intuitive interface, our research teams were able to confidently navigate the complexities of the trial to ensure all data elements were captured efficiently. Medidata has also helped reduce the administrative burden of data collection allowing the research teams to focus on patient outcomes. I look forward to working with Medidata [Professional Services] on future trials..”
– Jenna Brager PhD, RN, Executive VP, Drug Development, MyMD Pharmaceuticals, Inc.
“The Medidata RTSM Professional Services team has been outstanding in their efforts to support our business needs. Their Live Study Management (LSM) service has been invaluable, as we have a dedicated RTSM SME resource who can guide us in using RTSM throughout the entire live study phase and can address our RTSM questions. They will also make data changes in RTSM on our behalf as an unblinded resource. Their expertise and quality of service have been extremely beneficial to our study team and instrumental to our study’s success.”
Benefits of Rave RTSM
Unification of Rave RTSM and Rave EDC
Rave RTSM is fully unified with Rave EDC with no customized integrations, no reconciliation required and is fully configurable and pre-validated. The Rave RTSM and EDC solution is a paradigm shift from IRT/EDC as separate vendor systems. Sites record patient visits in Rave EDC as Rave RTSM assigns randomization arms and inventory items in real time. There is no need for double entry which increases the overall data integrity and reduces risk.
Abbreviated Timelines
Rave RTSM can be configured and implemented in as little as 2 weeks. Its pre-validated capabilities enable a quick UAT with reduced risk.
For mid-study changes, study builders can modify the study using RTSM’s state of the art Edit Live Design which enables changes to occur in real-time without stopping enrollment.
Shorten the Duration from LPI to Database Lock
Rave RTSM and Rave EDC are completely unified meaning there are no customized integrations reducing risk and timelines.
Data between RTSM and EDC requires no reconciliation as is required for a separate IRT and EDC model. This enables studies to lock much quicker, resulting in considerable cost savings.
Reduction of Risk
Rave RTSM is configurable and pre-validated. This means that RTSM works the same for all studies. What differs from one study to the next are the configuration settings of Randomization, Dispensation and Supply Management. With RTSM, User Acceptance Testing (UAT) is abbreviated with a smaller percentage of findings.
RTSM solution’s reduced risk is the biggest differentiator against all other IRT vendors.