Remote Monitoring in CNS Studies: The Power of Sensors
Reading Time: 3 minutesEffectively and accurately measuring disease progression in central nervous system (CNS)… Read More

COVID-19 Regulatory Developments Impacting Technology Use in Clinical Trials
Reading Time: 11 minutesNote: This post has been updated on October 20, 2020 to incorporate updated… Read More

Five Ways to Manage Your Trial Effectively in a Fast-Changing Global Environment
Reading Time: 4 minutesOver the past half-century, clinical trials have grown increasingly complex. A… Read More

In Information Security, Data Privacy, And Quality We Trust
Reading Time: 4 minutesWatch our webinar Unified Platform Defined available on-demand to learn the five… Read More

Medidata to Join Forces with Dassault Systèmes, Creating the First End-to-End Scientific and Business Platform for Life Sciences
Reading Time: < 1 minutesAccelerating the digital transformation for Life Sciences has been our… Read More

NEXT Hackathon 2018 Recap
This year’s NEXT NYC conference at Spring Studios was a resounding success—featuring speakers from Medidata, Amazon Web Services, Google Cloud, the Biden Cancer Initiative (with former Second Lady Jill Biden herself), and much more—catering to a diverse audience of over one thousand life sciences professionals during the two-day event. Read More

Closing the Gap on Gender Parity in the Workforce
"With the new day comes new strength and new thoughts." — Eleanor Roosevelt Read More

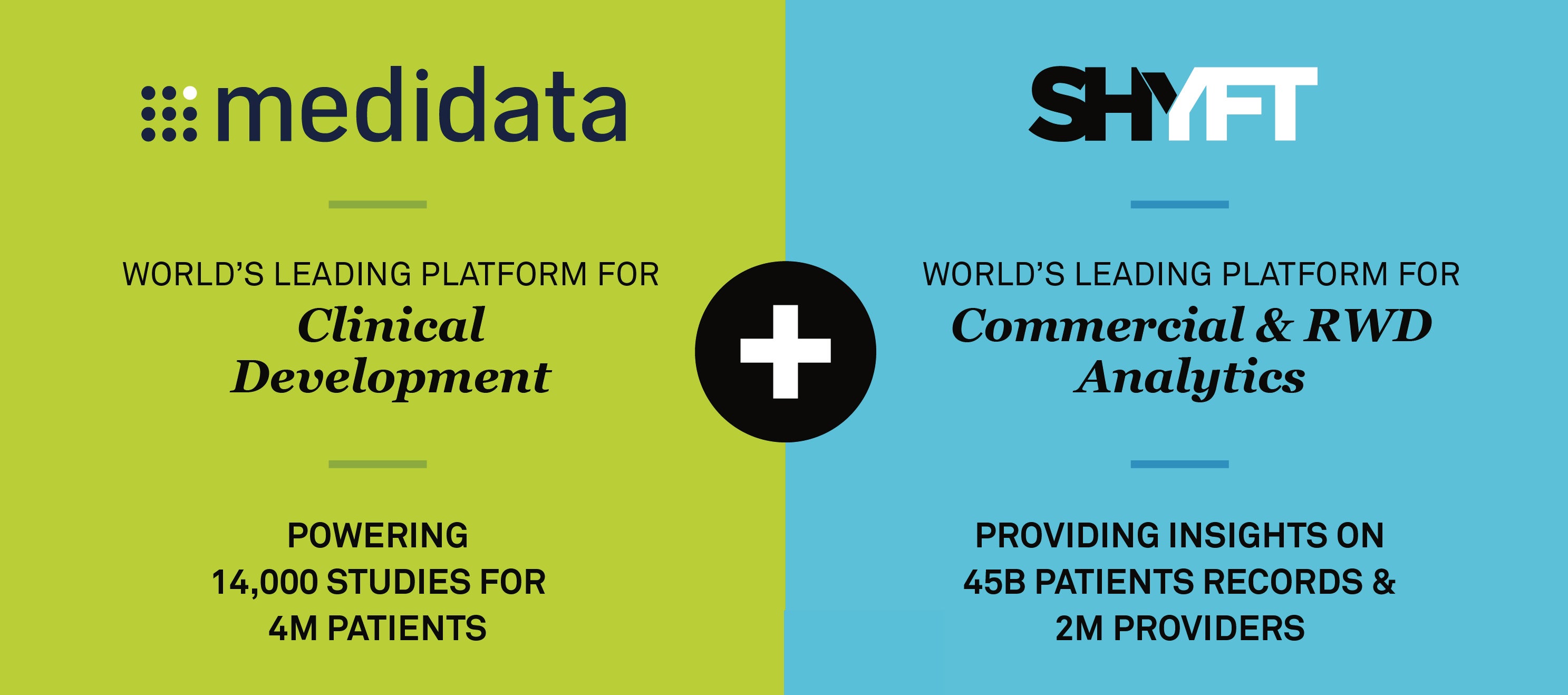
Medidata acquires SHYFT: Powering the Digital Transformation for Life Sciences
Digital transformation: this is one of the hottest topics in our industry today, creating both opportunities and challenges for our customers. For pharmaceutical, biotech, and medical device companies, the promise of making data-driven, insights-led decisions in real-time means getting the right treatments to the right patients at the right time. To truly realize this potential, they need to rely on combined insights from both clinical and commercial data. Read More
The Rewards and Pitfalls of Orphan Drug Development: PhaseBio, Medidata’s 1,000th Customer, Rises to the Challenge
PhaseBio—a clinical-stage biopharma company developing improved biotherapeutics for orphan diseases, with an initial focus on cardiopulmonary disorders—has the distinction of being Medidata's 1,000th customer. Read More
Taking the Risk Out of Clinical Trial Submissions using AI
So far Edge Trial Assurance has been used in 30 trials. It has detected 50-75 data anomalies in every single trial. Read More
