Medidata Supports World Cancer Day
Together, let’s solve the impossible for cancer research.


Medidata Helps in the Fight Against Cancer
Each year on February 4, the global community comes together for World Cancer Day to raise awareness about the disease and its impact on the patients who suffer from cancer and the families and friends who support them. The day acts as a powerful reminder that we all have a role to play in reducing the global impact of cancer.
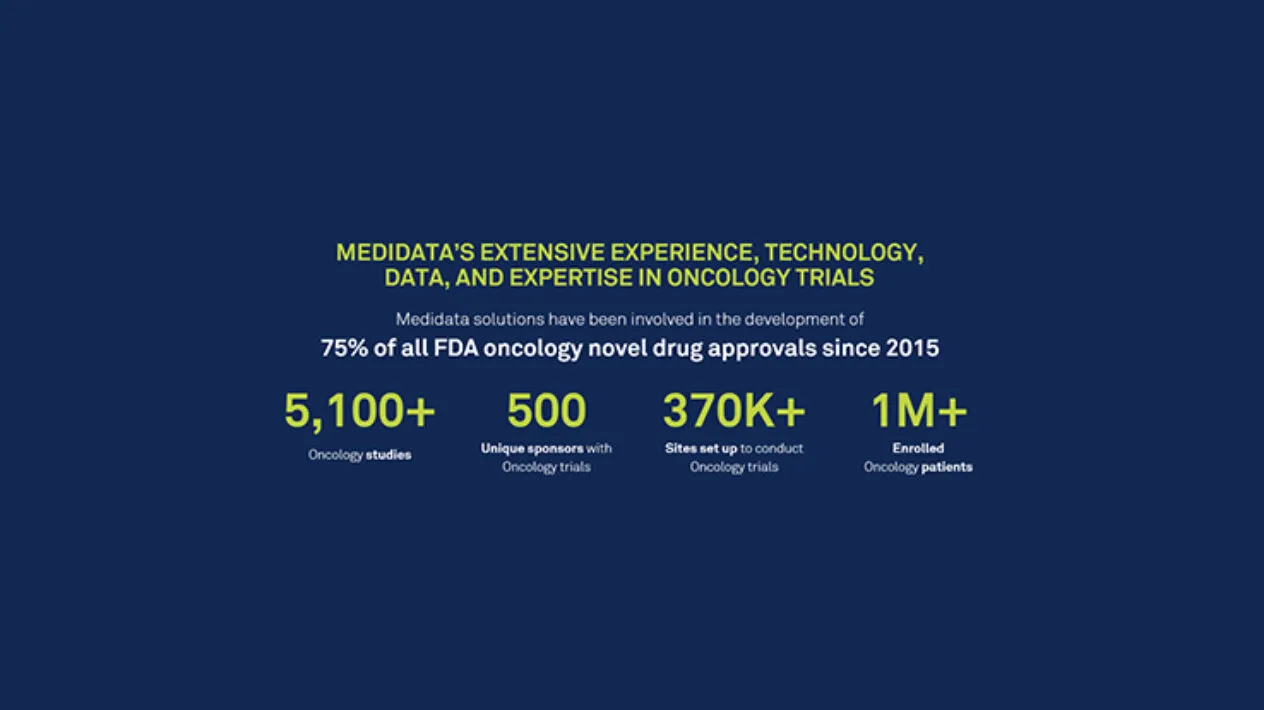
Cancer Facts
Cancer has a major impact on society across the world. Here are the facts*:
-
1 in 3 people will be diagnosed with cancer in their lifetime
-
Cancer is the second-leading cause of death worldwide, killing 10 million people every year
-
At least one third of common cancers are preventable
-
Millions of lives could be saved each year by implementing resource appropriate strategies for prevention, early detection and treatment
*https://www.worldcancerday.org/what-cancer