Historical clinical trials data can reduce the need for control arm patients
Summary
Recruiting patients for randomized control trials, particularly in small patient populations, represents a challenge for site investigators and life science company trial sponsors.[1]
From the patient perspective, an investigational drug can offer a new treatment option, particularly in rare and life threatening diseases. The chance of being in a control arm can be a disincentive for patients looking for investigational therapies and is a main reason patients choose not to participate in trials.[2] Additionally, if patients find out they are in a control arm, they may drop out or elect to receive therapies outside of the trial protocol.[3]
These factors complicate the decision process for patients, and they represent risks to a trial’s retention, completion, and valid statistical conclusions.
Medidata partnered with Friends of Cancer Research to find a solution to these recruitment and retention challenges and reduce the patient burden associated with randomized controls. The working group demonstrated that a synthetic control arm (SCA)TM — leveraging advanced analytics and patient-level data from multiple historical clinical trials — can mimic the results of a traditional randomized control. The results hold promise in finding ways to reduce the number of patients needed for a randomized control arm.
Methodology
Explore whether historical clinical trials data and matching procedures can stand in for prospectively randomized patients.
Project data came from:
- Project Data Sphere: Patient-level data from the control arms of three large randomized trials in non-small cell lung cancer (NSCLC)[4]
- Medidata Solutions: Patient-level data from multiple clinical trials in NSCLC conducted by the pharmaceutical industry for purposes of drug development, which are available in the Medidata Enterprise Data Store
Friends of Cancer Research, Medidata, members of the pharmaceutical industry, academia and patient groups created an SCA by selecting historical patients who met eligibility criteria and provided the same composition of baseline characteristics as patients in the investigational arm according to propensity score matching methods.
What is a propensity score? A propensity score is a commonly used epidemiology measure to balance the composition of groups at baseline to allow a fair comparison and support the conclusion that differences between the groups are due to the investigational therapy, not imbalances in baseline characteristics.
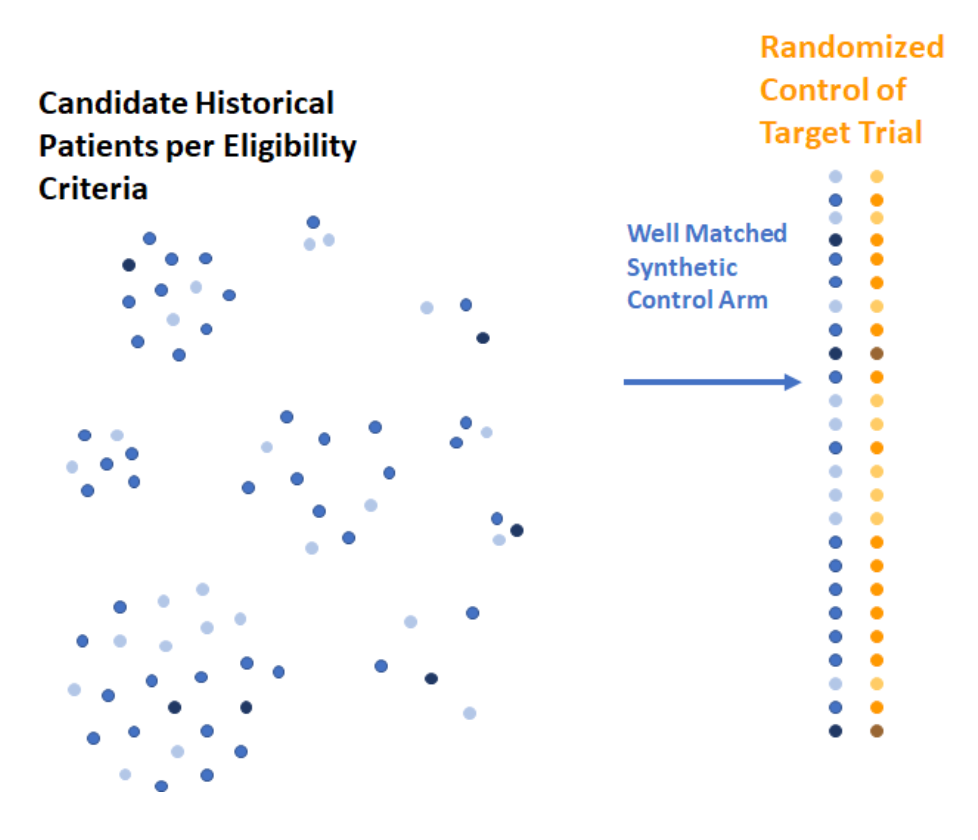
Left side: Patients for Synthetic Control Arm chosen from candidates that met eligibility criteria and were assigned to receive appropriate standard of care.
Right side: Synthetic Control Arm built based on patients with baseline characteristics that statistically match those in the randomized control.
Note: The intensity of color corresponds to various baseline characteristics
Key Findings
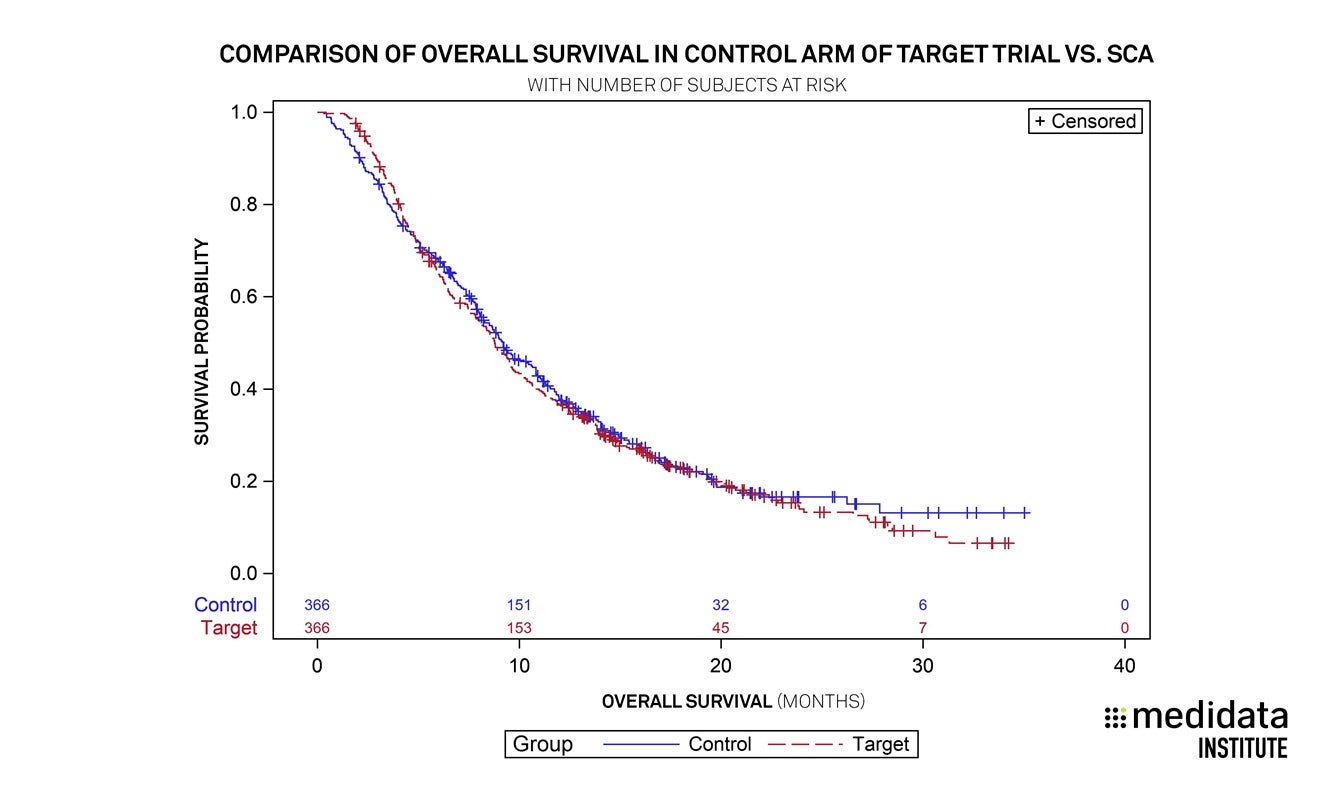
The propensity score matching successfully balanced the distribution of baseline characteristics between the SCA and the randomized control from the target trial, and overall survival for the SCA matched that of the randomized control in the target trial.
Implications
This analysis demonstrates it is possible to build a “matched” cohort, i.e. an external control arm, that replicates the outcomes of a randomized control arm by using propensity score matching and historical trial data.
Overall, the results are an important step toward understanding how synthetic control arms can minimize the number of patients required for randomized control arms. This approach may mitigate challenges associated with maintaining a concurrent control arm due to rarity of the disease or availability of the investigational agent outside the study. Minimizing the number of patients needed for a control arm reduces the burden on patients of a disease with high unmet need when the investigational therapy is perceived to offer better hope than the control arm’s standard therapy. Medidata has collaborated with academic institutions, regulatory agencies and advocacy organizations in previous endeavors, and its work in synthetic controls continues to expand.
Read the Friends of Cancer Research white paper to learn more.
[1] Augustine, E. F., Adams, H. R., & Mink, J. W. (2013). Clinical trials in rare disease: challenges and opportunities. Journal of child neurology, 28(9), 1142-50
[2] The American Cancer Society Cancer Action Network. Barriers to Patient Enrollment in Therapeutic Clinical Trials for Cancer: A Landscape Report. www.acscan.org/sites/default/files/National%20Documents/Clinical-Trials-Landscape-Report.pdf. 2018
[3] Kemmler G, Hummer M, Widschwendter C, Fleischhacker WW. Dropout Rates in Placebo-Controlled and Active-Control Clinical Trials of Antipsychotic Drugs: A Meta-analysis. Arch Gen Psychiatry. 2005;62(12):1305–1312. doi:10.1001/archpsyc.62.12.1305
[4] Project Data Sphere is a platform where the research community can share historical patient level data from academic and industry Phase III cancer clinical trials. The analyses in this case study are at least partially based on research using information obtained from www.projectdatasphere.org, which is maintained by Project Data Sphere, LLC.