Clinical Data Science 101: The New Clinical Data Management

As the clinical trial data acquisition landscape expands, the industry requires new approaches to support the rapid pace and increasing diversity of data sources. Thus, it’s not surprising that the focus of clinical data management teams is shifting from reactive, exhaustive data cleaning to proactive, risk-based clinical data science.
This fundamental shift requires clinical data managers to upskill and transform into clinical data scientists and strategic advisors who spend less time reviewing individual data points and more time proactively deriving insights and meaning from data to ensure quality. Read on to learn how Medidata empowers clinical data managers to transform and become more efficient, effective, and expert.

Traditional clinical data management methods are no longer suitable due to the increasing volume of trial data. Clinical data managers are overburdened as they face more data to review and clean and more queries to raise and resolve. One analysis of 20 Phase III studies reported that ~1.9 million queries were generated (averaging 97,000 per study). Additionally, although the absolute amount of electronic data capture (EDC) data is growing, it accounts for only ~30% of total study data. Data is now coming from a greater variety of sources—such as eCOA/ePRO, imaging, laboratory results, wearable sensors, and electronic health records—at an accelerating volume and velocity. The shortage of skilled clinical data managers is exacerbating the situation.
How can technology help clinical data science evolve in this rapidly developing environment? Medidata views the following three pillars as critical for this transformation:
- A centralized, 360° view of patient data
- Artificial intelligence that keeps clinical data managers in the loop
- Augmenting skill sets to transition to clinical data science
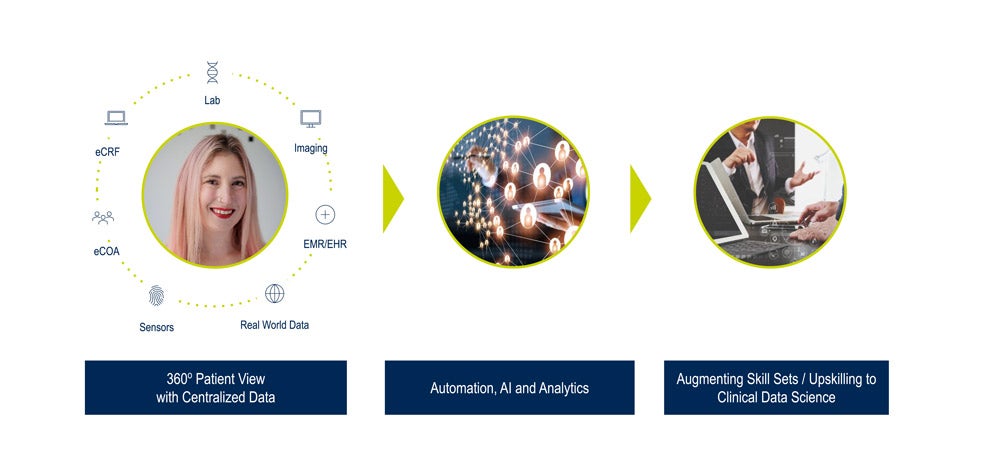
A Centralized, 360° View of Patient Data
Consolidating all trial data into one centralized source in near real-time is essential to develop an up-to-date and comprehensive view of the patient journey. Centralizing the data is especially important because data streams are becoming more varied and arising from an increasing number of external sources. Additionally, primary and secondary endpoints data are often measured from external data.
Building this 360° view of the patient can be accomplished by leveraging a unified platform, such as the Medidata Platform, that provides multiple, connected data acquisition experiences for patients and sites (including EDC, eConsent, eCOA/ePRO, Imaging, Sensors, EHR, and RTSM/IRT) and enables external (Sponsor/CRO/3rd-party system) data to be ingested and aggregated holistically for timely reviews in near real-time. The platform lets clinical data managers identify potential quality issues quickly and proactively across the entire clinical data spectrum. The data acquisition capabilities on Medidata’s unified platform significantly reduce the need to integrate various systems and execute data transfers that delay data availability.
With a centralized view of patient data, clinical data managers can perform their jobs more efficiently while considering all the data and gaining faster insights. The Medidata Platform supports this with robust clinical data management capabilities, where activities and workflows are driven from the full patient dataset. Clinical data managers have one, up-to-date workspace to aggregate, interrogate, review, and reconcile EDC and other data sources. Instead of navigating between multiple systems and trackers, discrepancies in data can be managed through a single interface that allows complex listings to be built, shared, and tracked—letting you generate and post multiple queries to the underlying EDC data. Configuring these powerful workflows is performed in a no-code environment, so data managers control their activities entirely, reducing reliance on clinical programming resources. These enhanced capabilities support upgrading practice from clinical data management to clinical data science.
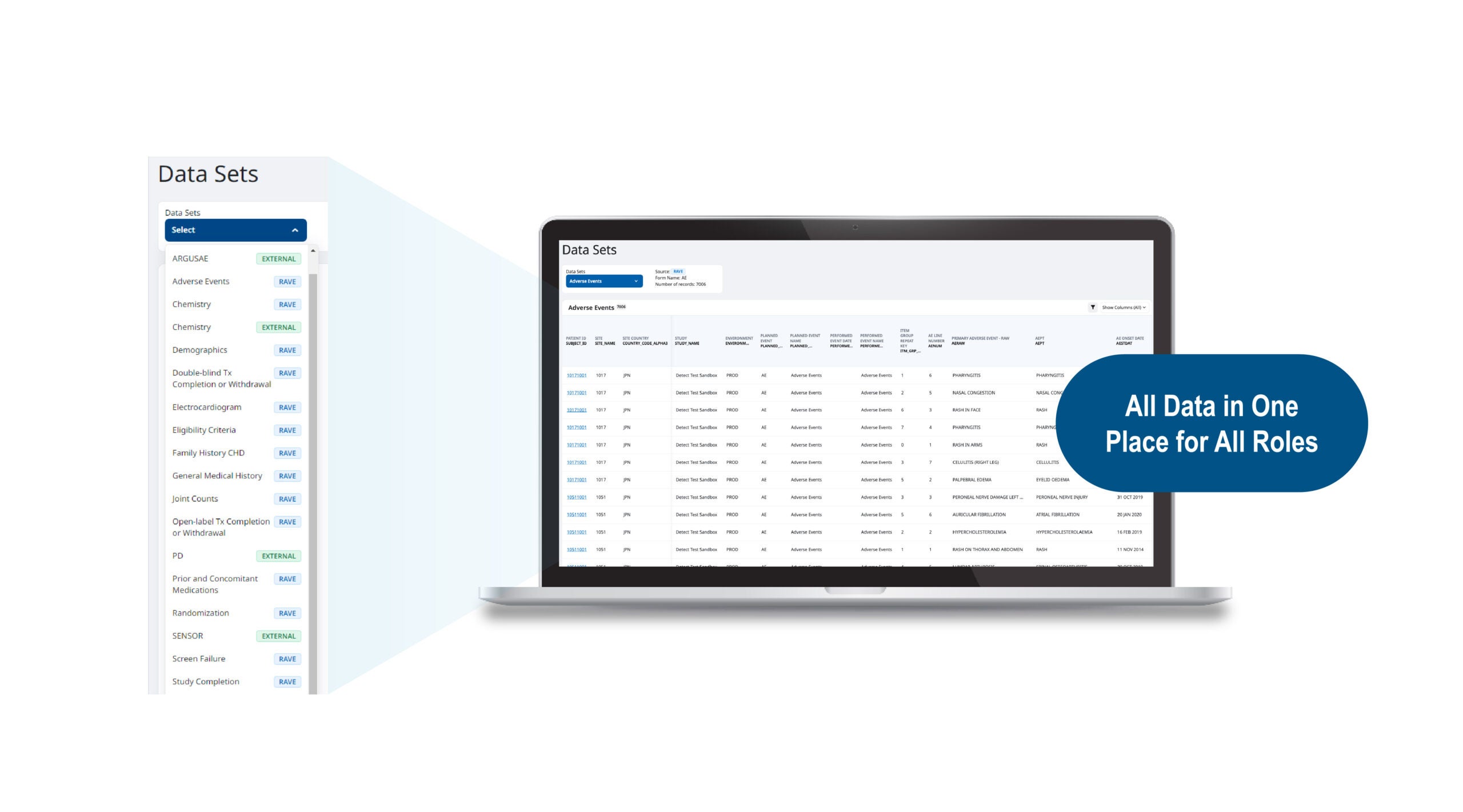
Together, the clinical data review capabilities of the Medidata Platform save considerable time that would otherwise be spent building listings across multiple external systems and performing manual query management. The Medidata Platform has been shown to reduce data review time per review cycle by up to 80%.
AI That Keeps Data Managers in the Loop
The second step of transforming clinical data management to clinical data science involves a shift toward a more intelligent data review supported by analytics and Artificial Intelligence (AI) that serves as a decision-support tool. These algorithms do not make final decisions but enhance productivity by highlighting potential discrepancies across complex datasets. For instance, the algorithm can rapidly and automatically identify and rank discrepancies from adverse events, medical history, and concomitant medication datasets and surface them for review and further investigation. The clinical data manager can assess the accuracy of the findings and provide feedback to the algorithm, continuously improving its performance. The clinical data manager fully controls the workflow, adjudicating outputs from the algorithm and taking appropriate action. Data managers can quickly review records and automatically submit queries based on the algorithm’s feedback. They can also perform deeper exploration to establish clinical context using integrated visual patient profiles and directly link to underlying data.
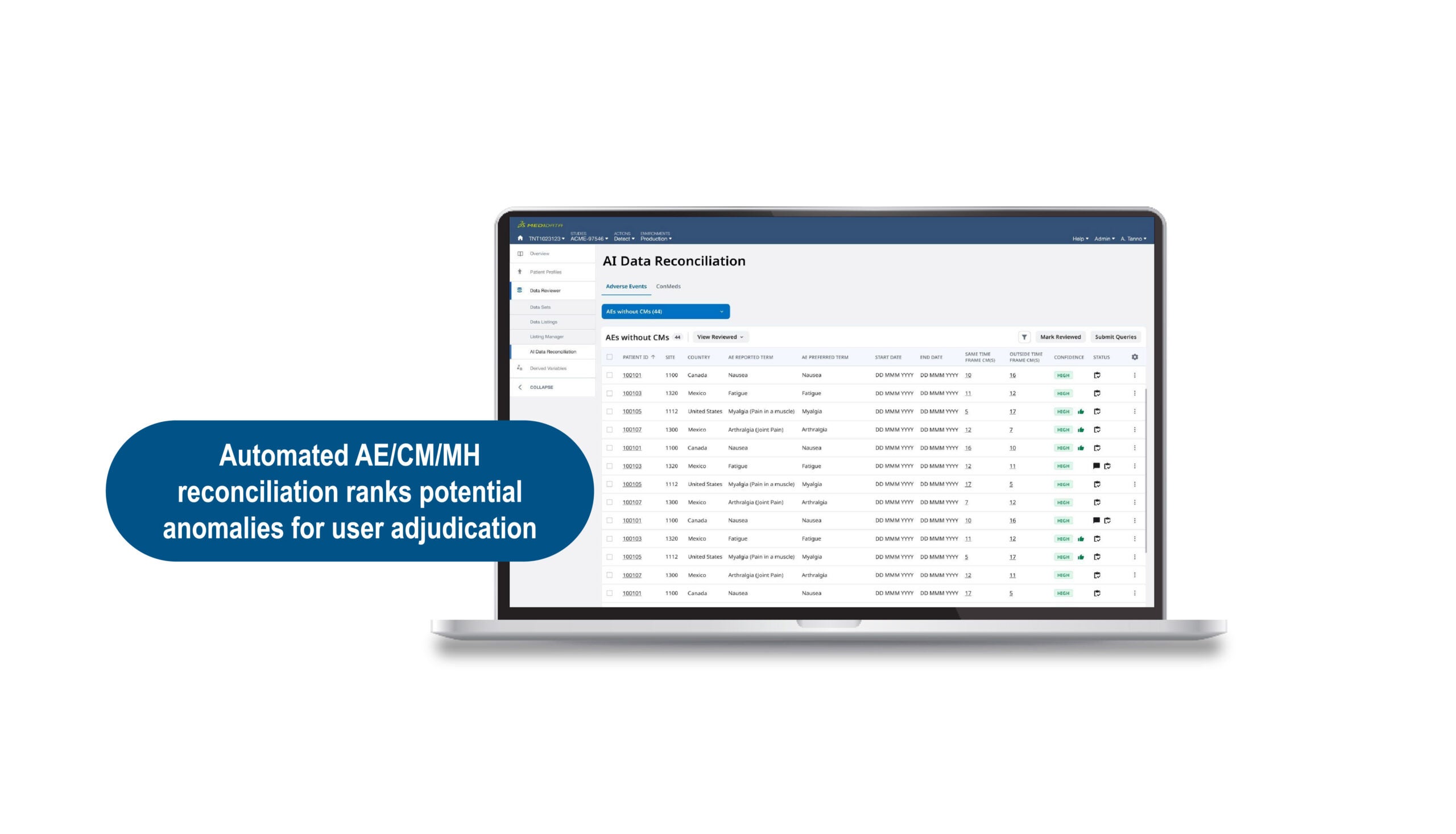
Medidata’s AI algorithms are trained and tested using the industry’s most extensive historical clinical trials dataset. By incorporating user input, these algorithms learn over time and improve their assistance for clinical data managers. AI provides a unique opportunity to de-risk data management activities and save time. It also supports the evolution of clinical data managers to clinical data scientists, who can spend less time on labor-intensive, manual data cleaning tasks and more time on data analysis and proactive quality management.
Upskilling of Clinical Data Management to Clinical Data Science
The third step of the transformation involves upskilling clinical data management teams so they embrace AI algorithms as transformative tools in data management. Despite uncertainties related to AI, these technologies hold immense potential for boosting productivity and providing fresh perspectives into data analysis. But clinical data managers must educate themselves on the distinctions between AI and machine learning (ML) and how these technologies function to fully capitalize on this opportunity. This education will lead to a better understanding of how data managers can leverage these tools and empower them to actively participate in the training of algorithms (which directly impacts their performance) and an understanding of different use cases of AI versus ML.
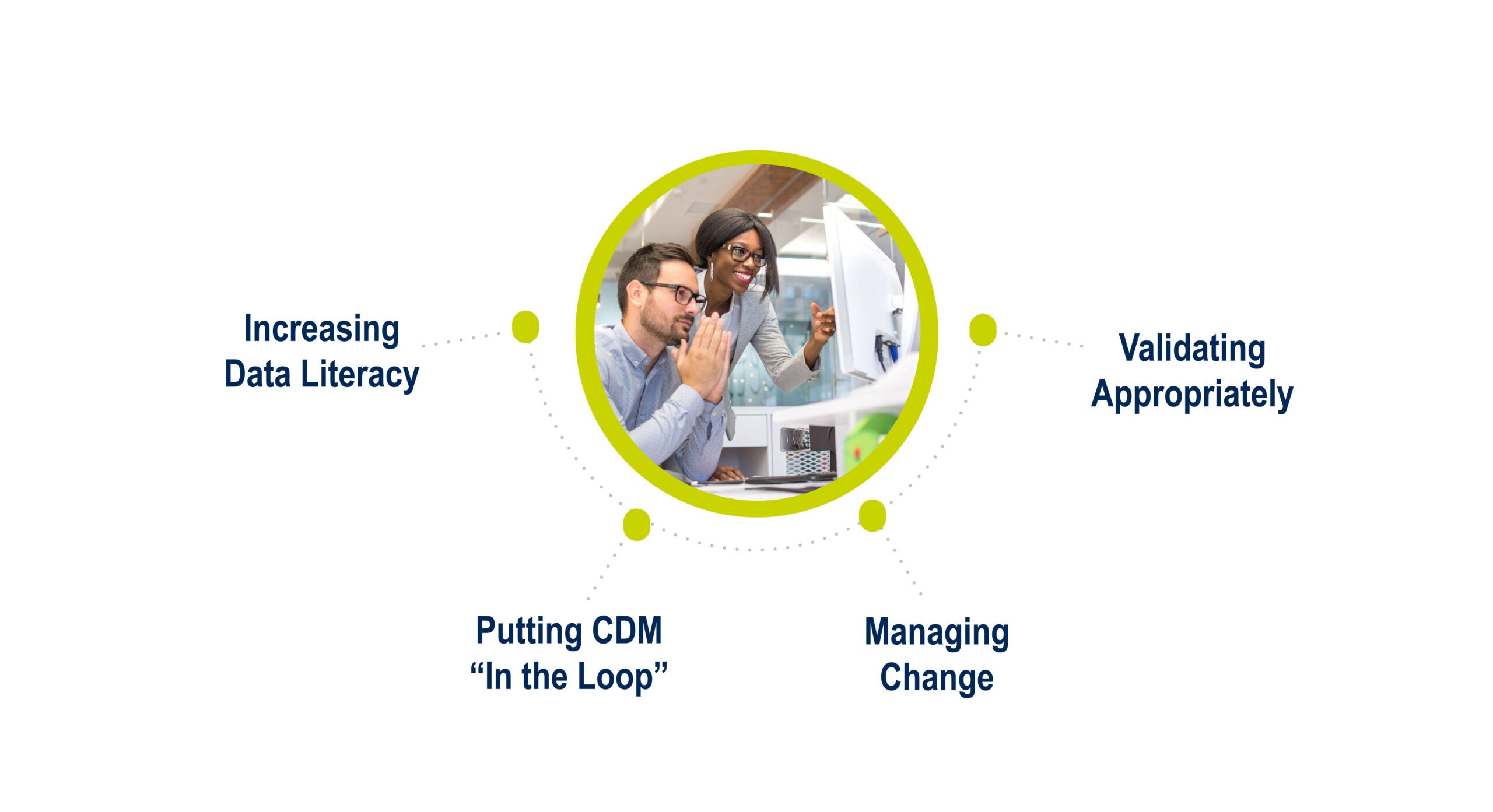
Upskilling presents an exceptional opportunity for data managers to elevate their roles to strategic contributors by actively validating algorithms, testing them across different datasets, and collaborating with vendors and regulators to ensure alignment in the adopted approach. Rather than fearing the uncertainties associated with these technologies, data managers can use this to broaden their skill set, play a more strategic role, and contribute significantly to transforming clinical data management to clinical data science.
Conclusion
Next-generation technologies empower the transformation from clinical data management to clinical data science. This evolution includes consolidating all trial data, irrespective of source, into one centralized source in near real-time. It combines technology-driven processes such as automation and AI (for data review and reconciliation) and enhanced data exploration using smart visualizations. It’s time to transform clinical data management to be more efficient and effective to meet the challenges of today’s and tomorrow’s clinical trials.
Watch our webinar hosted by the Society for Clinical Data Management (SCDM) to learn more about Medidata’s solutions for transforming clinical data management to clinical data science.
Explore Related Articles
Contact Us


