Preparing for a New Data Future: Perceptions of Unified Platform Deployment Strategy
This blog is part two of a five-part series on the findings of this survey of clinical research technology decision makers. Download Preparing for a New Data Future: A Survey of Clinical Research Technology Decision Makers for comprehensive survey results to guide your unified platform approach to clinical trials.
A rapid shift towards virtualization due to the COVID-19 pandemic, larger volumes of data collection from an increasing number and diverse set of data sources, and the need for sponsors and CROs to gain more complete pictures of trials and to act on data quickly are forcing the industry towards eClinical software solutions that are on unified platforms. Our new survey of key decision makers in clinical research technology examined industry outlook on deployment strategy of eClinical platforms. Key findings include:
Companies are increasingly deploying eClinical software solutions via the cloud
Based on data from several interviews, ~70–80% of large life science companies’ eClinical software capabilities are cloud hosted. Respondents indicated broad acceptance of cloud hosting as fit for purpose and suitable for compliance, privacy, data ownership, and auditing. Replacing legacy software remains the primary limiting factor.
Cloud solutions remove IT overhead
IT departments seeking to eliminate unnecessary costs are removing large and complex infrastructure and instead deploying cloud-based solutions primarily hosted and supported by external vendors.
The shift is tougher for large incumbents
Life science tech leaders now expect cloud-hosted SaaS solutions, though larger sponsors take longer to replace vast legacy infrastructure that supports point solutions. They require significant change management, SOP updates, and new vendor and data management processes to move over to a fully outsourced cloud-hosted SaaS solution.
New organizations looking first to the cloud
Cloud-based solutions allow for easier cross-organization working and tighter control of data flow for newly formed or smaller, more nimble organizations.
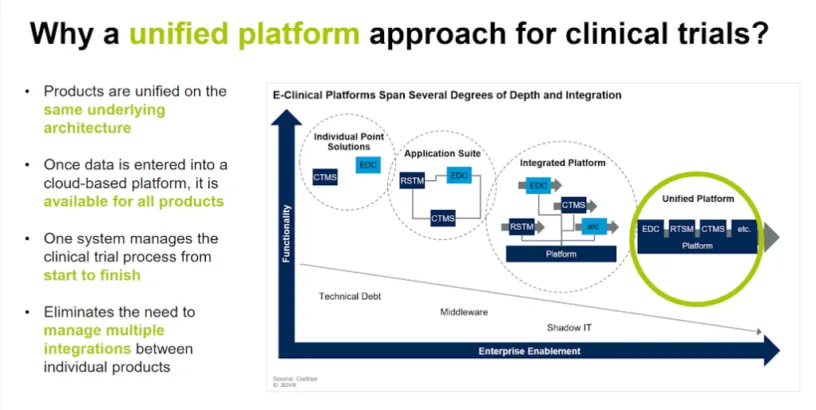
The pharmaceutical industry will continue to evolve and adapt to its increasingly digital environment and move from single and fragmented point solutions to unified platforms. Embracing unified platforms and cloud integration eases the burden on clinical development teams, scales to support large numbers of studies, and allows sponsors to centralize their data to gain powerful insights across trials.
Stay tuned for the next edition of this five-part series. Download Preparing for a New Data Future: A Survey of Clinical Research Technology Decision Makers for comprehensive survey results to guide your unified platform approach to clinical trials.
Contact Us
