Rave Clinical Operations
Action Powered by Data and Advanced Analytics
Medidata is the only provider capable of taking 25+ years of data and insights and operationalizing it across the trial continuum, moving you beyond static, siloed systems, to an ecosystem providing a complete view of the patient and site experience, and unified workflows driving faster trial timelines.
Medidata powers study teams with technology that spans the trial lifecycle – Trial Execution and Oversight, Risk-Based Quality Management, and Clinical Trial Financial Management – and accelerates workflows by embedding analytics across clinical processes.
Medidata transforms clinical operations workflows through the Medidata Platform, the industry’s only unified platform dedicated to clinical research. By centralizing your data and clinical operations technology on a single platform, you can move from insight to action more quickly.
Why Rave Clinical Operations
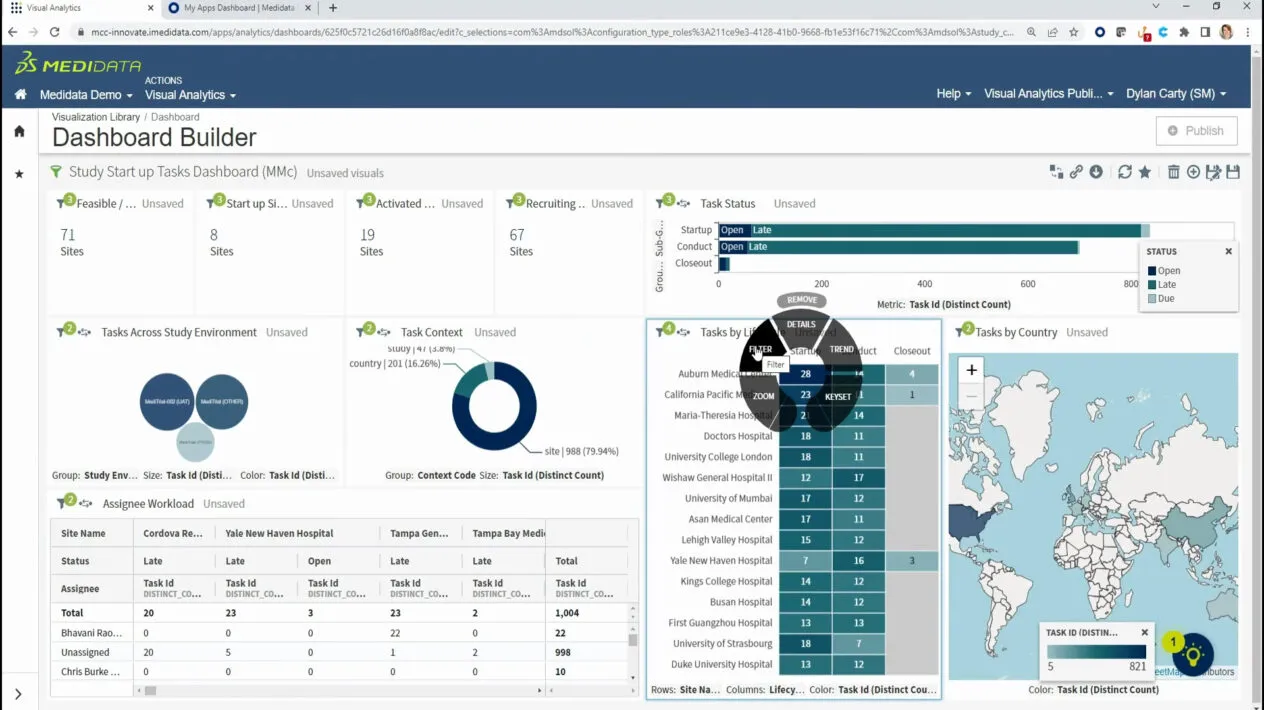
Drive Faster Clinical Trial Decisions with Data
“Data first” drives everything we do at Medidata. We enable optimized clinical trial operations by surfacing actionable data insights to target and focus study team activities. We achieve this through our 10+ year investment in Data Science, embedding powerful analytics where they are needed most – within core clinical operations workflows – bringing insight directly to the frontlines of decision-making.

Improve Clinical Trial Oversight
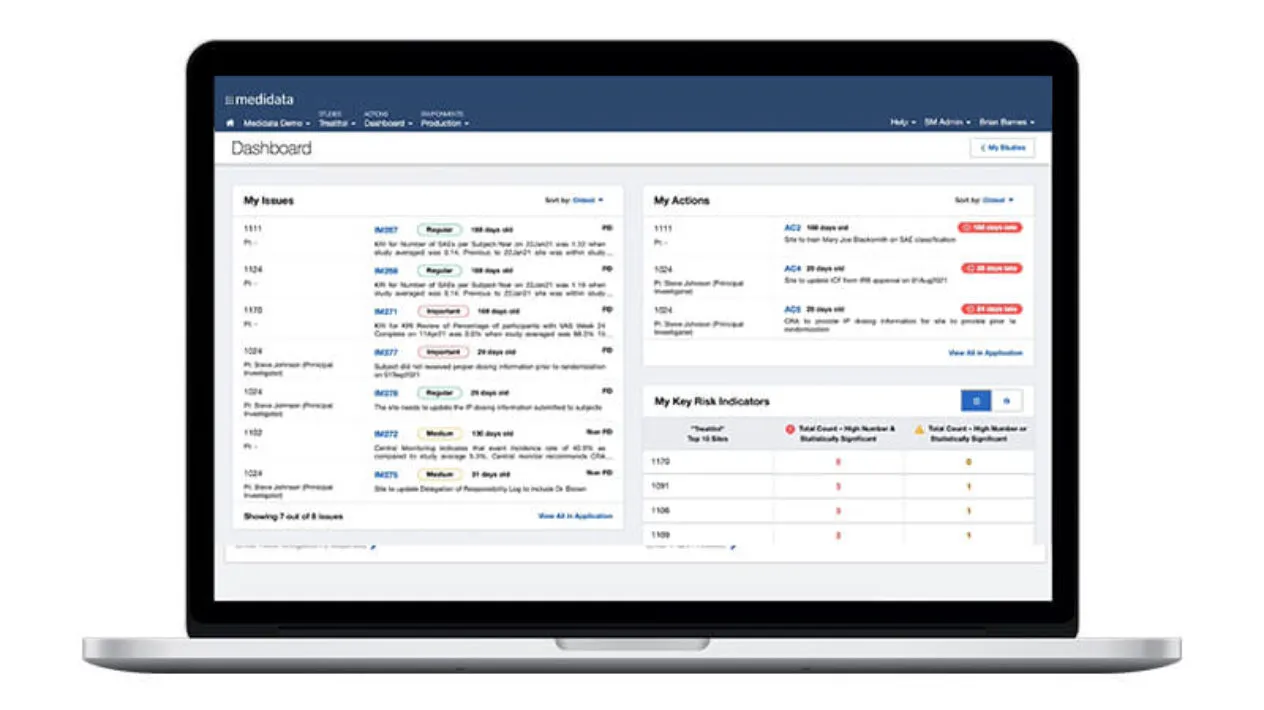
The growing complexity of the clinical trial ecosystem highlights the need to maintain continuous oversight at both the study and program level. Regardless of the outsourcing model, organizations need to manage trial risk, site performance, data integrity, and regulatory compliance. True oversight goes beyond visibility: it provides the power to remediate issues in time to avoid trial delays. Medidata delivers holistic, truly digital oversight through coordinated capabilities built on aggregated data.
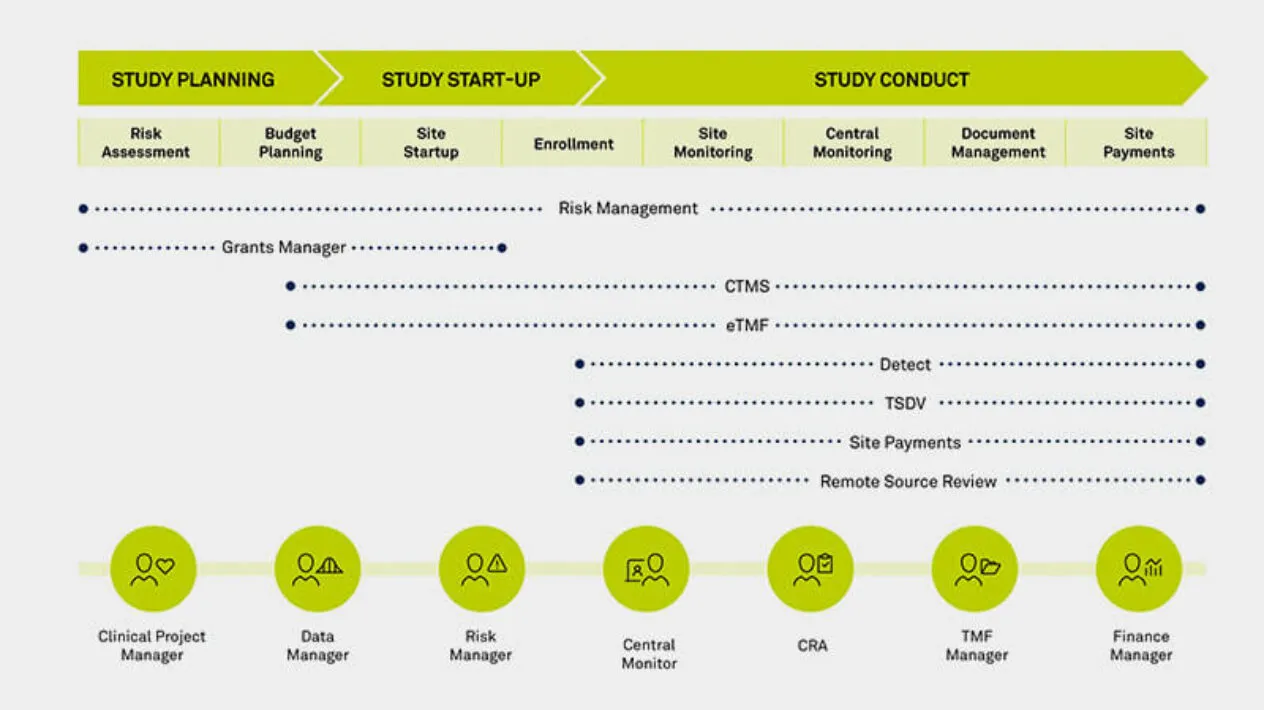
Connect Users Across the Clinical Trial Lifecycle
As the functional silos within clinical teams break down, so too must the boundaries between clinical technologies break down. From trial planning through execution, Medidata provides a single data ecosystem for users to interact across capabilities. Now your teams across clinical operations, data management, monitoring, risk management, and finance all have a common environment to communicate with each other, complete workflows bridging across applications, and foster collaboration across their teams.
Accelerate the New Generation of Decentralized Clinical Trials
Today’s clinical trials are faster, have vastly more data, much of it collected directly from the patient via multiple modalities, often outside of traditional investigative sites. Medidata is the only provider that offers a comprehensive decentralized clinical trial (DCT) solution that allows sponsors and CROs to prepare for and analyze high volumes of data at scale. Now, you can move from delayed, reactive methodologies to proactive and predictive strategies that drive better, faster decisions.
Rave Clinical Operations Solutions

Clinical Trial Execution and Oversight
Accelerate study startup, improve monitoring efficiency, optimize physical and virtual interactions with sites, and remain constantly inspection-ready, in an analytics-powered ecosystem for digitally executing and overseeing clinical studies.

Risk-Based Quality Management (RBQM)
When implemented properly, adopting risk-based quality management strategies can shorten trial timelines, reduce overall costs, and improve trial outcomes. Medidata provides the technology and expertise to successfully transform your approach to clinical operations towards risk-based study execution models.

Clinical Trial Financial Management
Ensure the financial health of your trials by connecting the dots between the study budget planning, site budget negotiation and site payment processes within a single access point for all stakeholders. Make the complex, simple.

Medidata Clinical Operations Services
Medidata deploys our deep internal expertise to manage key clinical processes for you, reducing FTE burden and improving ROI. Our experts work for you to ensure your processes are optimized, your business is transformed, and you attain the maximum value out of Medidata technologies.
Customer Success Stories
Solving the Impossible for COVID-19 with Medidata Technology
“We understood and knew that this data analytics solution, Detect, could enable us with identifying the various or potential data errors and trends within the data. This would then allow us to improve data integrity and reduce the trial risk.”
—
Laurie Callen
Senior Director of Clinical Data Management
Moderna

TD2 Partners with Medidata to Enhance its Clinical Operations and Accelerate Clinical Trials
“The addition of Medidata’s CTMS and eTMF platforms will allow for even more standardization across data management and clinical operations, creating efficiency, while reducing integration time and resources.”
—
Dr. Stephen Gately
President and CEO
TD2

Regenerative Medicine Pioneer Issues Site Payments Accurately, on Time, and Transparently
“Medidata has become an extension of our team. The Site Payments service streamlined the payment process from end to end, benefiting both our company and our sites.”
—
Nick McCoy
Associate Vice President for Clinical Operations
BioTissue