Archive: 2022
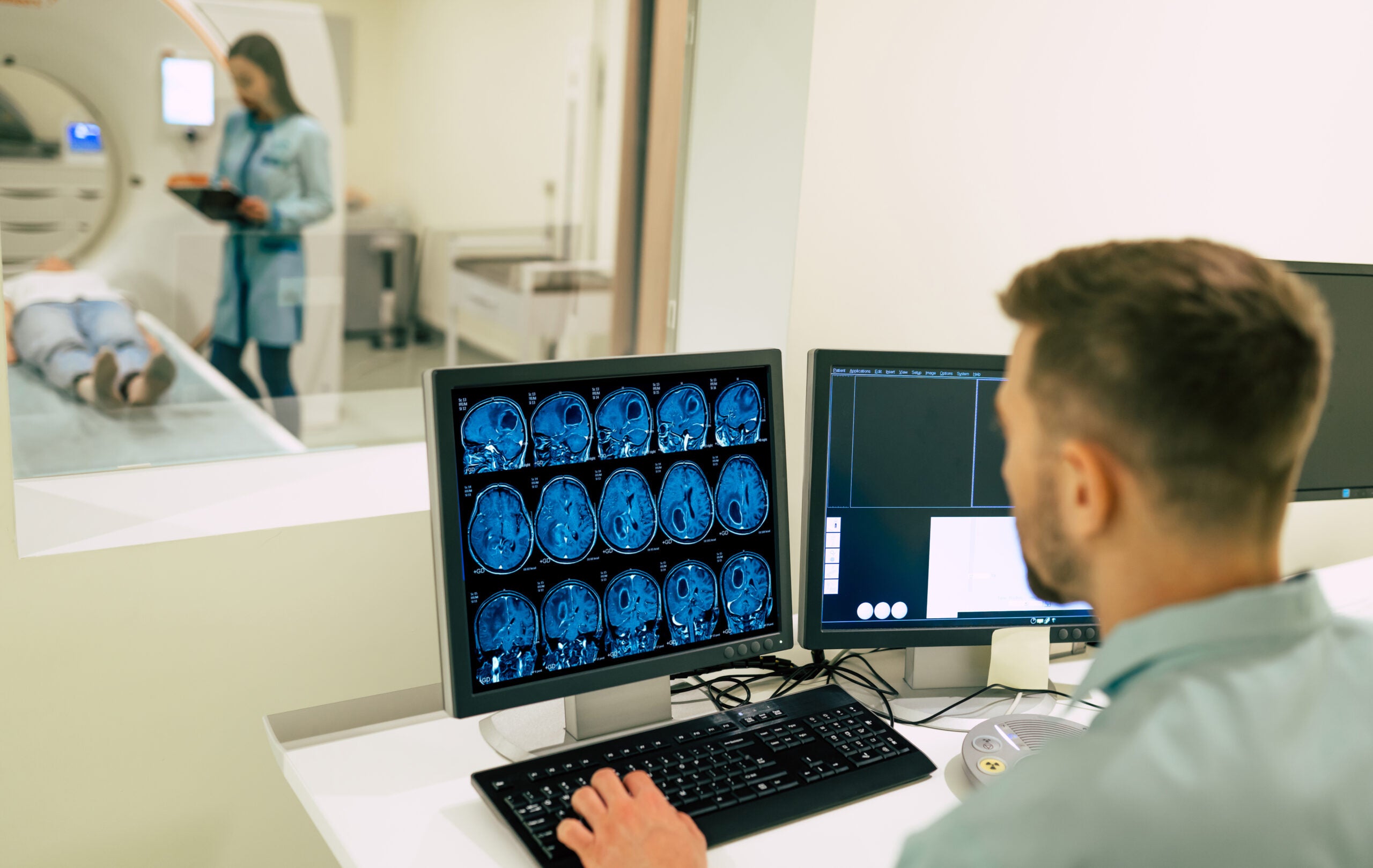
How to Take Your Imaging Trials to the Next Level with Automation and AI
Reading Time: 3 minutesThe use of medical imaging in clinical research is becoming more… Read More
Medidata AI Selected to Present at National Comprehensive Cancer Network (NCCN) Annual Conference
Reading Time: 2 minutesMedidata AI is committed to consistently redefining how collaborative clinical trial… Read More

Bridging the Gap: Connecting with Life Sciences Employees in a COVID-19 World
Reading Time: 3 minutesThis blog was authored by Mieke Whaley, Director, EMEA… Read More

RBQM in Its Prime: Companies Realize Lasting Benefits When Adopting Risk-based Approaches
Reading Time: 2 minutesThe landscape of clinical trials has changed significantly in the last… Read More

Electronic Signatures – A Brief Overview of the EU Landscape
Reading Time: 3 minutesThis blog was authored by Fiona Maini, Global Compliance… Read More

Revolutionizing Patient Engagement in Clinical Trials | A Medidata NEXT Global Series
Reading Time: 3 minutesMedidata’s annual event series—NEXT Global—brought together leaders from across the life… Read More

AI-powered Insights in Clinical Trials | A Medidata NEXT Global Series
Reading Time: 3 minutesMedidata’s annual event series—NEXT Global—brought together leaders from across the life… Read More

Medidata’s Rave EDC Solution: All About Value
Reading Time: 3 minutesThis blog was authored by Katrina Weigold, Vice President… Read More

Improving Ulcerative Colitis Treatment Using the Mayo Score and Electronic Clinical Outcomes Assessment in Clinical Trials
Reading Time: 3 minutesThe Mayo Score has been a long-established assessment tool within clinical… Read More

Patient Insights from Sensor Data in Clinical Trials – The Context that Makes Movies Out of Moments | Patient Perspectives
Reading Time: 3 minutesThis blog was authored by Anne Marie Mercurio. Anne Marie is… Read More
