Complex Clinical Trials: Strategies for Success Amidst Evolving Methodologies

This blog was authored by Christopher Tyers, Client Services Principal at Medidata.
At November’s Medidata NEXT New York 2023, 500+ clinical trial sponsors and partners came together with a focus on the betterment of complex clinical trials. The synergy of their collective experience, expertise, thought leadership, and shared insights led to industry-shaping discussions and a greater awareness of new possibilities that lie ahead.
Discussions frequently centered on finding ways to address ever-increasing complexity within the industry. During the Innovative Approaches for Tackling Complex Trials session moderated by Steve Evon, Vice President of Global Clinical Operations Solution Sales, he asked a panel of industry specialists how clinical trials have changed in complexity over the past 10–15 years.
As a whole, the industry has worked tirelessly to reduce burdens across the ecosystem through technology, processes, and attempts at standardization. Despite this, complexity has continued to increase year-on-year. What’s driving this increased complexity?
At a high level, three areas stand out: protocols, operations, and data.
Defining Complex Clinical Trials
Complex trial design, or complex innovative trial design, describes several methodologies; the FDA and EMA provide subtly different descriptions (Figure 1).

1. Protocol Complexity – The Increasing Use of Complex Trial Design Methods
Figure 1. What is Complex Trial Design?
Complex trial designs are methodologies that include adaptive trials (adaptive, basket, dose-ranging, platform, or umbrella), targeted/stratified, and Bayesian methods. Their continued adoption has moved beyond usage in traditionally complex studies, such as oncology and rare diseases, and is now used across other therapeutic areas.
The benefits and rewards of these methods are compelling; these include an increased probability of study success while using fewer resources, and the capability of allowing a parallel investigation of multiple treatments for several diseases or patient groups, both within an overall shorter timescale.
“We have complex trials because we have many objectives for each trial, and each objective is complex in and of itself.”
– Miganush Stepanians, Ph.D., President and CEO, PROMETRIKA
These methods are more efficient but involve increased complexity from operational, data, and analysis perspectives, presenting technical challenges—often insurmountable for many legacy systems and current disparate technology offerings in terms of a lack of flexibility, scalability, and interoperability. Considering the many areas within a study that are impacted, this is by no means a small issue.
A few examples of increases that impact complexity are shown in Figure 2, published by Tufts Centre for the Study of Drug Development in July/August 2018: operational increases in procedures (70%), investigative sites (63%), protocol complexity with an increase in endpoints (86%), and data complexity from an increase in data points collected (88%).
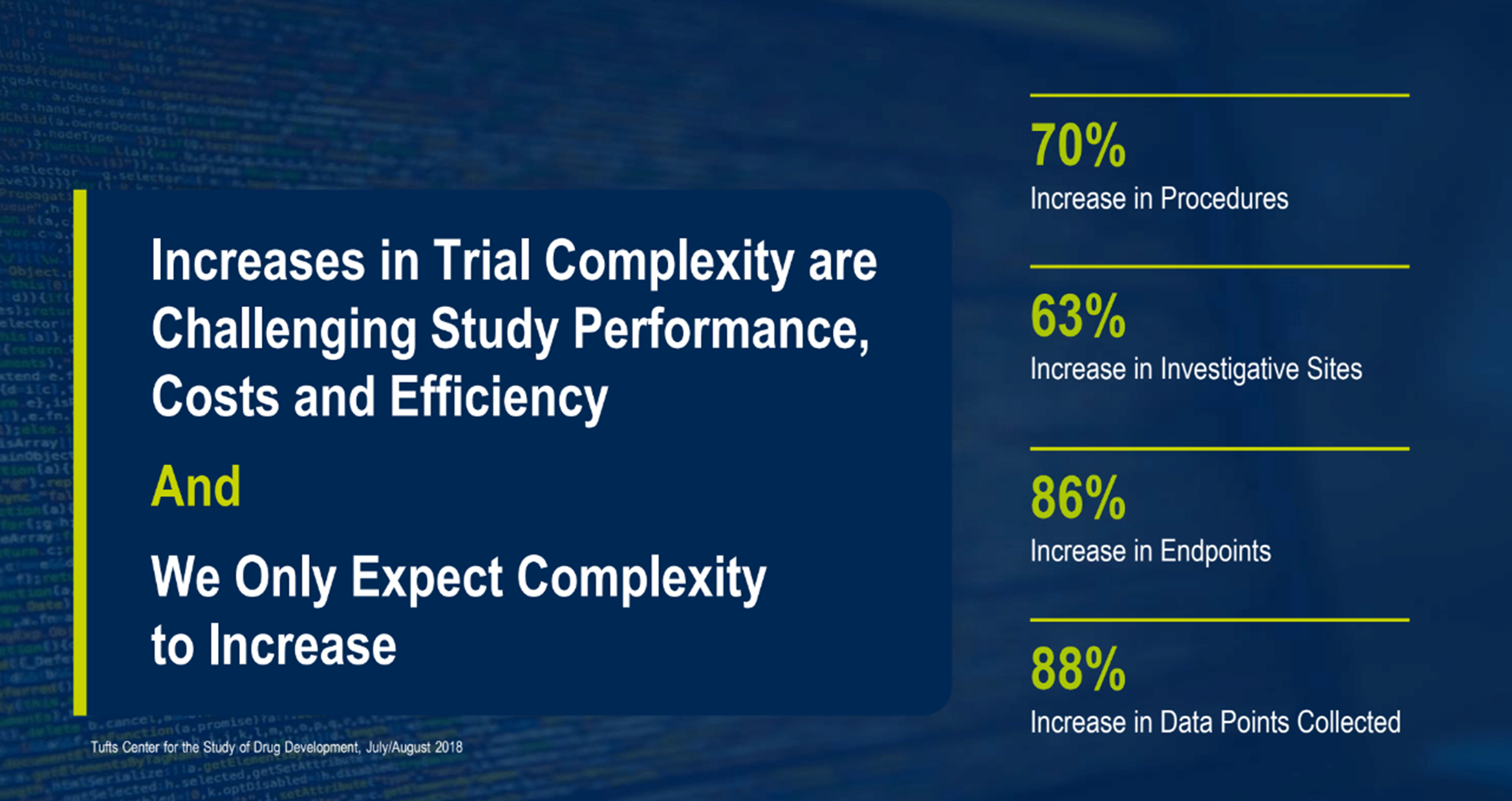
Figure 2. Increases in Trial Complexity.
While the statistics are from 2018, the upward trajectory in complexity has been widely acknowledged since then, and forecasts show that it will continue on an accelerated path. Kenneth Getz, the frequently quoted executive director of Tufts, spoke at the Drug Information Association (DIA) Global Annual Meeting in June 2023, commenting on the continued growth trajectory again.
2. Operational Complexity – The Multiple Layers of Clinical Trials
Managing Global Studies
Operationally, an increase in global studies has a massive impact on the challenges of planning, budgeting, forecasting, supply chain management, local and national site management, and patient recruitment and retention. A multitude of localized requirements and considerations must be taken into account, including translations, adoption of new technology, training, adapting to decentralized trials (DCTs), and the logistics of delivering/returning resources to sites and subjects.
A common challenge in the later stages of planning and forecasting global studies is the lack of finalized information, such as the actual number of sites involved, and which countries will be included in the study.
Decentralized Trials (DCT)
With the industry shift to DCTs, there have been positive reports of fewer protocol amendments, faster recruitment, and higher retention. Equally though, the adoption of DCTs has brought about complexities through the implementation and integration of new technology and processes to support DCT activities, such as home visits, direct-to-patient drug deliveries/collection, and delivery/collection of devices.
Consider this for one study—it’s complex. Now expand that to global studies with high levels of uncertainty, challenges, multiple systems, multiple vendor collaboration requirements, etc. The level of complexity has grown exponentially; without the right partners in the process to address all of these challenges, any lead time and efficiency advantages may be lost.
“...from the sponsor side, finding a vendor who knows how to work with systems that can handle complex trials is really important.”
– Monica Massaro, Senior Director, Biostatistics, Synlogic Therapeutics
Technology and Innovation – The Double-Edged Sword
Independent technology providers bring new solutions to the market every year to address specific challenges—supply management or site payments for example—and therein lies part of the problem. In isolation, many do it well within their specific scope. The challenge for sponsors when adopting independent solutions is invariably in terms of interoperability, data management, integration, implementation, scalability, device management, training, support, and burdens on partners and patients—especially when considering global studies.
As one example, at the roundtable session following the complexity panel discussion, representatives from research sites brought up the challenges of the impact on studies when using disparate systems for complex trials. The issues that they face are well documented, with a clear need for a unified approach. If they have to use disparate systems, each system has its login, user interface, operating procedures, and variable levels of training and support, often leading to problems, delays, and pain for the sites that can negatively impact enrollment and retention. This is at the research site user level and does not even touch on the operational and technical challenges named above.
Technology should address problems, not create them.
So, should independent vendors be ignored? No. But, as all systems aren’t created equal, assessing their ability to address your specific challenges is critical. The saying, “too many cooks in the kitchen spoil the broth” is very appropriate here.
Working with a partner that has an established, trusted, and unified platform of proven solutions with vetted partners is the best option to address these challenges.
“...a major key to achieving these difficult goals in one trial is to have the right technology that you apply to that trial.”
– Miganush Stepanians, Ph.D., President and CEO, PROMETRIKA
3. Data Management in Complex Trials
Complex trial design results in an increased number of endpoints and higher volumes of data and analysis. Challenges increase exponentially across the spectrum of data management processes, including the collection of excess data, isolating gaps, managing duplication, integration with systems, and interoperability.
The interoperability challenge alone costs the healthcare industry over $30 billion per year in the US. This topic is discussed in more detail in this recent article.
A unified platform addresses these challenges, enabling decreased build time and increased efficiencies, flexibility, and scalability. The other key advantage of a unified system is that data analytics tools work on a full dataset that resides in real-time from a single source, enabling visibility of quickly identifiable outliers and trends that can be actioned immediately.
As highlighted by Dr. Stepanians, a major advantage of a complex trial methodology is the ability to make changes to the structure along the way. A unified platform allows for faster change management and traceability between functions when quick changes are needed.
Again, using a unified platform with established partners and solid interoperability with critical data sources, such as electronic health records (EHRs) and key partner systems, is a significant step forward in addressing data complexity and challenges.
“...when you build the EDC and the RTSM on the same platform by the same team, that in fact could potentially save some time on the overall build. So I think that's a big plus.”
– Benny Chan, Senior Biostatistical Standards and Implementation Specialist, PROMETRIKA
In Summary
By their very nature, clinical trials are complex. Addressing this has always been the challenge, and now, with the increased adoption of complex clinical trial methods, increased operational complexity, and unprecedented real-world global issues affecting us all, that challenge has never been greater.
With the multitude of highly complex layers within a successful study, finding the right technology, services, and expertise is not always straightforward. Choosing the wrong partner brings risks that impact the entire study. To avoid this, it’s important to make sure your chosen partner possesses the depth and breadth of expertise necessary to help you mitigate risks and manage your study with best practices learned from extensive industry experience.
“...you need to have the right technology, the integrated platform that Medidata offers, but also the right experts. The expertise in-house at Medidata, [and also the partner] who executes the trial for you, need to have done this before in pieces or [as a] whole. And I think it's a combination of all of that that will make it a success. So I would not attempt a complex trial with elementary technology and inexperienced folks. It won't be a good outcome.”
– Miganush Stepanians, Ph.D., President and CEO, PROMETRIKA
Learn how to build the right tech stack for adaptive trial designs, hybrid protocols, and more:
Explore Related Articles
Contact Us


